当前位置:
X-MOL 学术
›
Sustain. Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanical activation in reduced graphite oxide/boron nitride nanocomposite electrocatalysts for significant improvement in dioxygen reduction
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7se00461c Indrajit M. Patil 1, 2, 3, 4, 5 , Chamundi P. Jijil 4, 6, 7, 8 , Moorthi Lokanathan 1, 2, 3, 4, 9 , Anita Swami 2, 3, 4, 5 , Bhalchandra Kakade 1, 2, 3, 4, 5
Sustainable Energy & Fuels ( IF 5.0 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1039/c7se00461c Indrajit M. Patil 1, 2, 3, 4, 5 , Chamundi P. Jijil 4, 6, 7, 8 , Moorthi Lokanathan 1, 2, 3, 4, 9 , Anita Swami 2, 3, 4, 5 , Bhalchandra Kakade 1, 2, 3, 4, 5
Affiliation
|
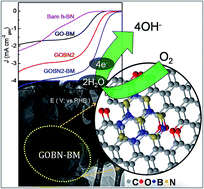
A nanocomposite of reduced graphite oxide (rGO) with hexagonal boron nitride (h-BN) is prepared using a simple hydrothermal method. Significant enhancement in the surface area of the composite is mainly due to the pre-mechanical activation of pristine GO. The structural and morphological study reveals the formation of a homogeneous nanocomposite and masking of rGO sheets over micron sized h-BN particles respectively. Interestingly, the as-synthesized GOBN2–BM composite (nanocomposite of 2 wt% h-BN with mechanically activated GO) catalyst exhibits significant oxygen electroreduction kinetics in terms of onset potential (Eonset = 0.89 V), half-wave potential (E1/2 = 0.74 V) and limiting current density (JL = 4.4 mA cm−2) with a single step ∼4-electron transfer pathway in alkaline medium. Though the composite catalysts exhibit higher overpotential (110 mV) than state-of-the-art Pt/C catalysts, they are much superior to previously reported carbon or h-BN based nanocomposite electrocatalysts. Importantly, the GOBN2–BM nanocomposite shows excellent tolerance towards both methanol oxidation and CO poisoning. Moreover, the nanocomposite catalysts show substantially higher stability than Pt/C catalysts even after 5000 cycles under similar conditions. Additionally, they show a better relative current stability (95% retention) than that of a Pt/C catalyst, signifying immense selectivity and durability towards oxygen electroreduction kinetics. The electrocatalytic oxygen reduction activity of the nanocomposite is mainly attributed to the high surface area (thanks to mechanical activation of GO, leading to increased pore distribution) as well as the synergistic mechanism between the h-BN and carbon network of rGO. Hence, it could be a potential cathode catalyst to replace precious-metal based catalysts in alkaline fuel cells.
中文翻译:

还原的氧化石墨/氮化硼纳米复合电催化剂中的机械活化,可显着改善双氧还原
使用简单的水热法制备了具有六方氮化硼(h-BN)的还原型氧化石墨(rGO)的纳米复合材料。复合材料表面积的显着提高主要归因于原始GO的预机械活化。结构和形态研究揭示了均匀的纳米复合材料的形成和rGO片在微米级h-BN颗粒上的掩蔽。有趣的是,刚合成的GOBN2-BM复合材料(含2 wt%h-BN的纳米复合材料和机械活化的GO)催化剂在起始电势(E起始= 0.89 V),半波电势(E 1)方面显示出显着的氧电还原动力学。/ 2 = 0.74 V)和极限电流密度(J L = 4.4 mA cm−2)在碱性介质中只有一步〜4电子转移途径。尽管复合催化剂比现有的Pt / C催化剂具有更高的超电势(110 mV),但它们比以前报道的基于碳或h-BN的纳米复合电催化剂优越得多。重要的是,GOBN2-BM纳米复合材料对甲醇氧化和一氧化碳中毒显示出优异的耐受性。而且,即使在相似条件下循环5000次之后,纳米复合催化剂也显示出比Pt / C催化剂显着更高的稳定性。此外,它们显示出比Pt / C催化剂更好的相对电流稳定性(95%保留),表明对氧电还原动力学具有极大的选择性和持久性。纳米复合材料的电催化氧还原活性主要归因于高表面积(由于GO的机械活化,导致孔分布增加)以及h-BN与rGO碳网络之间的协同机制。因此,它可能是替代碱性燃料电池中贵金属基催化剂的潜在阴极催化剂。
更新日期:2017-11-13
中文翻译:

还原的氧化石墨/氮化硼纳米复合电催化剂中的机械活化,可显着改善双氧还原
使用简单的水热法制备了具有六方氮化硼(h-BN)的还原型氧化石墨(rGO)的纳米复合材料。复合材料表面积的显着提高主要归因于原始GO的预机械活化。结构和形态研究揭示了均匀的纳米复合材料的形成和rGO片在微米级h-BN颗粒上的掩蔽。有趣的是,刚合成的GOBN2-BM复合材料(含2 wt%h-BN的纳米复合材料和机械活化的GO)催化剂在起始电势(E起始= 0.89 V),半波电势(E 1)方面显示出显着的氧电还原动力学。/ 2 = 0.74 V)和极限电流密度(J L = 4.4 mA cm−2)在碱性介质中只有一步〜4电子转移途径。尽管复合催化剂比现有的Pt / C催化剂具有更高的超电势(110 mV),但它们比以前报道的基于碳或h-BN的纳米复合电催化剂优越得多。重要的是,GOBN2-BM纳米复合材料对甲醇氧化和一氧化碳中毒显示出优异的耐受性。而且,即使在相似条件下循环5000次之后,纳米复合催化剂也显示出比Pt / C催化剂显着更高的稳定性。此外,它们显示出比Pt / C催化剂更好的相对电流稳定性(95%保留),表明对氧电还原动力学具有极大的选择性和持久性。纳米复合材料的电催化氧还原活性主要归因于高表面积(由于GO的机械活化,导致孔分布增加)以及h-BN与rGO碳网络之间的协同机制。因此,它可能是替代碱性燃料电池中贵金属基催化剂的潜在阴极催化剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号