Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin's lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin Study Group.
The Lancet ( IF 98.4 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1470-2045(17)30696-4 Dennis A Eichenauer , Annette Plütschow , Stefanie Kreissl , Martin Sökler , Johannes C Hellmuth , Julia Meissner , Stephan Mathas , Max S Topp , Karolin Behringer , Wolfram Klapper , Georg Kuhnert , Markus Dietlein , Carsten Kobe , Michael Fuchs , Volker Diehl , Andreas Engert , Peter Borchmann
The Lancet ( IF 98.4 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1470-2045(17)30696-4 Dennis A Eichenauer , Annette Plütschow , Stefanie Kreissl , Martin Sökler , Johannes C Hellmuth , Julia Meissner , Stephan Mathas , Max S Topp , Karolin Behringer , Wolfram Klapper , Georg Kuhnert , Markus Dietlein , Carsten Kobe , Michael Fuchs , Volker Diehl , Andreas Engert , Peter Borchmann
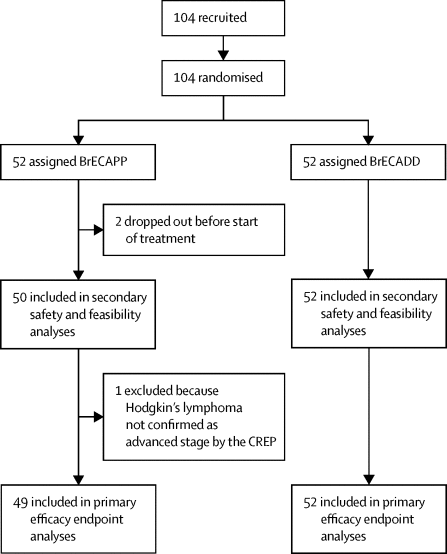
|
A high proportion of patients with relapsed classical Hodgkin's lymphoma achieve a response with the antibody-drug conjugate brentuximab vedotin, and the drug is well tolerated. We modified the escalated BEACOPP regimen (eBEACOPP; bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone) and implemented brentuximab vedotin with the aim to reduce toxic effects while maintaining the protocol's efficacy.
中文翻译:

将brentuximab vedotin纳入一线治疗晚期经典霍奇金淋巴瘤:德国霍奇金研究小组对一项2期随机试验的最终分析。
很大一部分复发的经典霍奇金淋巴瘤患者可通过抗体-药物结合物brentuximab vedotin产生应答,并且该药物具有良好的耐受性。我们修改了逐步升级的BEACOPP方案(eBEACOPP;博来霉素,依托泊苷,阿霉素,环磷酰胺,长春新碱,普卡巴嗪和泼尼松),并实施了brentuximab vedotin,目的是在维持方案疗效的同时降低毒性作用。
更新日期:2017-11-30
中文翻译:

将brentuximab vedotin纳入一线治疗晚期经典霍奇金淋巴瘤:德国霍奇金研究小组对一项2期随机试验的最终分析。
很大一部分复发的经典霍奇金淋巴瘤患者可通过抗体-药物结合物brentuximab vedotin产生应答,并且该药物具有良好的耐受性。我们修改了逐步升级的BEACOPP方案(eBEACOPP;博来霉素,依托泊苷,阿霉素,环磷酰胺,长春新碱,普卡巴嗪和泼尼松),并实施了brentuximab vedotin,目的是在维持方案疗效的同时降低毒性作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号