Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-11-10 , DOI: 10.1016/s0140-6736(17)32409-1 Sonia S Anand 1 , Jackie Bosch 2 , John W Eikelboom 1 , Stuart J Connolly 1 , Rafael Diaz 3 , Peter Widimsky 4 , Victor Aboyans 5 , Marco Alings 6 , Ajay K Kakkar 7 , Katalin Keltai 8 , Aldo P Maggioni 9 , Basil S Lewis 10 , Stefan Störk 11 , Jun Zhu 12 , Patricio Lopez-Jaramillo 13 , Martin O'Donnell 14 , Patrick J Commerford 15 , Dragos Vinereanu 16 , Nana Pogosova 17 , Lars Ryden 18 , Keith A A Fox 19 , Deepak L Bhatt 20 , Frank Misselwitz 21 , John D Varigos 22 , Thomas Vanassche 23 , Alvaro A Avezum 24 , Edmond Chen 25 , Kelley Branch 26 , Darryl P Leong 1 , Shrikant I Bangdiwala 27 , Robert G Hart 1 , Salim Yusuf 1 ,
The Lancet ( IF 98.4 ) Pub Date : 2017-11-10 , DOI: 10.1016/s0140-6736(17)32409-1 Sonia S Anand 1 , Jackie Bosch 2 , John W Eikelboom 1 , Stuart J Connolly 1 , Rafael Diaz 3 , Peter Widimsky 4 , Victor Aboyans 5 , Marco Alings 6 , Ajay K Kakkar 7 , Katalin Keltai 8 , Aldo P Maggioni 9 , Basil S Lewis 10 , Stefan Störk 11 , Jun Zhu 12 , Patricio Lopez-Jaramillo 13 , Martin O'Donnell 14 , Patrick J Commerford 15 , Dragos Vinereanu 16 , Nana Pogosova 17 , Lars Ryden 18 , Keith A A Fox 19 , Deepak L Bhatt 20 , Frank Misselwitz 21 , John D Varigos 22 , Thomas Vanassche 23 , Alvaro A Avezum 24 , Edmond Chen 25 , Kelley Branch 26 , Darryl P Leong 1 , Shrikant I Bangdiwala 27 , Robert G Hart 1 , Salim Yusuf 1 ,
Affiliation
|
BACKGROUND
Patients with peripheral artery disease have an increased risk of cardiovascular morbidity and mortality. Antiplatelet agents are widely used to reduce these complications.
METHODS
This was a multicentre, double-blind, randomised placebo-controlled trial for which patients were recruited at 602 hospitals, clinics, or community practices from 33 countries across six continents. Eligible patients had a history of peripheral artery disease of the lower extremities (previous peripheral bypass surgery or angioplasty, limb or foot amputation, intermittent claudication with objective evidence of peripheral artery disease), of the carotid arteries (previous carotid artery revascularisation or asymptomatic carotid artery stenosis of at least 50%), or coronary artery disease with an ankle-brachial index of less than 0·90. After a 30-day run-in period, patients were randomly assigned (1:1:1) to receive oral rivaroxaban (2·5 mg twice a day) plus aspirin (100 mg once a day), rivaroxaban twice a day (5 mg with aspirin placebo once a day), or to aspirin once a day (100 mg and rivaroxaban placebo twice a day). Randomisation was computer generated. Each treatment group was double dummy, and the patient, investigators, and central study staff were masked to treatment allocation. The primary outcome was cardiovascular death, myocardial infarction or stroke; the primary peripheral artery disease outcome was major adverse limb events including major amputation. This trial is registered with ClinicalTrials.gov, number NCT01776424, and is closed to new participants.
FINDINGS
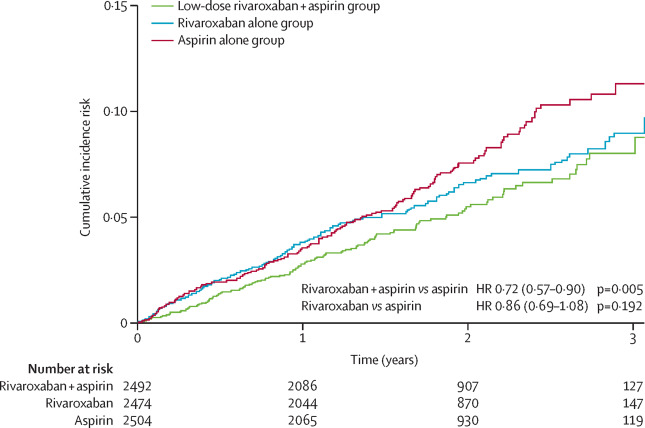
Between March 12, 2013, and May 10, 2016, we enrolled 7470 patients with peripheral artery disease from 558 centres. The combination of rivaroxaban plus aspirin compared with aspirin alone reduced the composite endpoint of cardiovascular death, myocardial infarction, or stroke (126 [5%] of 2492 vs 174 [7%] of 2504; hazard ratio [HR] 0·72, 95% CI 0·57-0·90, p=0·0047), and major adverse limb events including major amputation (32 [1%] vs 60 [2%]; HR 0·54 95% CI 0·35-0·82, p=0·0037). Rivaroxaban 5 mg twice a day compared with aspirin alone did not significantly reduce the composite endpoint (149 [6%] of 2474 vs 174 [7%] of 2504; HR 0·86, 95% CI 0·69-1·08, p=0·19), but reduced major adverse limb events including major amputation (40 [2%] vs 60 [2%]; HR 0·67, 95% CI 0·45-1·00, p=0·05). The median duration of treatment was 21 months. The use of the rivaroxaban plus aspirin combination increased major bleeding compared with the aspirin alone group (77 [3%] of 2492 vs 48 [2%] of 2504; HR 1·61, 95% CI 1·12-2·31, p=0·0089), which was mainly gastrointestinal. Similarly, major bleeding occurred in 79 (3%) of 2474 patients with rivaroxaban 5 mg, and in 48 (2%) of 2504 in the aspirin alone group (HR 1·68, 95% CI 1·17-2·40; p=0·0043).
INTERPRETATION
Low-dose rivaroxaban taken twice a day plus aspirin once a day reduced major adverse cardiovascular and limb events when compared with aspirin alone. Although major bleeding was increased, fatal or critical organ bleeding was not. This combination therapy represents an important advance in the management of patients with peripheral artery disease. Rivaroxaban alone did not significantly reduce major adverse cardiovascular events compared with asprin alone, but reduced major adverse limb events and increased major bleeding.
FUNDING
Bayer AG.
中文翻译:

利伐沙班有或无阿司匹林治疗稳定的外周或颈动脉疾病的患者:一项国际,随机,双盲,安慰剂对照试验。
背景技术患有外周动脉疾病的患者患心血管疾病和死亡的风险增加。抗血小板药被广泛用于减少这些并发症。方法这是一项多中心,双盲,随机安慰剂对照试验,从六大洲33个国家的602家医院,诊所或社区机构招募了患者。符合条件的患者有下肢周围动脉疾病的病史(先前的外周旁路手术或血管成形术,肢体或足部截肢,间歇性c行以及客观证据表明周围动脉疾病的病史),颈动脉病史(颈前动脉血运重建或无症状颈动脉狭窄至少50%),或踝臂指数小于0·90的冠状动脉疾病。经过30天的磨合期后,患者被随机分配(1:1:1)接受口服利伐沙班(每天2次,5毫克)和阿司匹林(每日一次100毫克),利伐沙班每天两次(5)每日一次与阿司匹林安慰剂合计1毫克),或每天一次与阿司匹林合用(每天两次,分别与100毫克利伐沙班安慰剂合用)。随机化是计算机产生的。每个治疗组都是双重假人,患者,研究人员和中心研究人员都无法进行治疗分配。主要结果是心血管死亡,心肌梗塞或中风。主要的外周动脉疾病预后为主要的不良肢体事件,包括大截肢。该试验已在ClinicalTrials.gov上注册,编号为NCT01776424,并且不对新参与者开放。发现在2013年3月12日至2016年5月10日之间,我们从558个中心招募了7470例外周动脉疾病患者。与单独使用阿司匹林相比,利伐沙班加阿司匹林的组合减少了心血管死亡,心肌梗塞或中风的复合终点(1492的[5%] [1492]与2504的[174] [7%];危险比[HR] 0·72、95) %CI 0·57-0·90,p = 0·0047),以及包括肢体截肢在内的严重肢体不良事件(32 [1%] vs 60 [2%]; HR 0·54 95%CI 0·35-0 ·82,p = 0·0037)。利伐沙班5 mg每天两次与单独使用阿司匹林相比,并未显着降低复合终点(2474的149 [6%]比2504的174 [7%]; HR 0·86,95%CI 0·69-1·08, p = 0·19),但减少了包括主要截肢在内的主要不良肢体事件(40 [2%] vs 60 [2%]; HR 0·67,95%CI 0·45-1·00,p = 0·05 )。中位治疗时间为21个月。与单独使用阿司匹林组相比,利伐沙班加阿司匹林联合使用可增加严重出血(2492的77 [3%]比2504的48 [2%]; HR 1·61,95%CI 1·12-2·31, p = 0·0089),主要是胃肠道。同样,在2474例利伐沙班5毫克患者中,有79例(3%)发生了大出血,而阿司匹林单独治疗组中2504例中有48例(2%)(HR 1·68,95%CI 1·17-2·40; p = 0·0043)。解释与单独使用阿司匹林相比,每天服用两次低剂量利伐沙班加阿司匹林可以减少主要的不良心血管和肢体事件。尽管大出血增加了,但致命或关键器官的出血却没有。这种联合疗法代表了在治疗周围动脉疾病患者方面的重要进展。与单独使用阿斯普林相比,单独使用利伐沙班并没有显着减少主要不良心血管事件,但是减少了主要不良肢体事件并增加了严重出血。资助拜耳公司。
更新日期:2018-01-19
中文翻译:

利伐沙班有或无阿司匹林治疗稳定的外周或颈动脉疾病的患者:一项国际,随机,双盲,安慰剂对照试验。
背景技术患有外周动脉疾病的患者患心血管疾病和死亡的风险增加。抗血小板药被广泛用于减少这些并发症。方法这是一项多中心,双盲,随机安慰剂对照试验,从六大洲33个国家的602家医院,诊所或社区机构招募了患者。符合条件的患者有下肢周围动脉疾病的病史(先前的外周旁路手术或血管成形术,肢体或足部截肢,间歇性c行以及客观证据表明周围动脉疾病的病史),颈动脉病史(颈前动脉血运重建或无症状颈动脉狭窄至少50%),或踝臂指数小于0·90的冠状动脉疾病。经过30天的磨合期后,患者被随机分配(1:1:1)接受口服利伐沙班(每天2次,5毫克)和阿司匹林(每日一次100毫克),利伐沙班每天两次(5)每日一次与阿司匹林安慰剂合计1毫克),或每天一次与阿司匹林合用(每天两次,分别与100毫克利伐沙班安慰剂合用)。随机化是计算机产生的。每个治疗组都是双重假人,患者,研究人员和中心研究人员都无法进行治疗分配。主要结果是心血管死亡,心肌梗塞或中风。主要的外周动脉疾病预后为主要的不良肢体事件,包括大截肢。该试验已在ClinicalTrials.gov上注册,编号为NCT01776424,并且不对新参与者开放。发现在2013年3月12日至2016年5月10日之间,我们从558个中心招募了7470例外周动脉疾病患者。与单独使用阿司匹林相比,利伐沙班加阿司匹林的组合减少了心血管死亡,心肌梗塞或中风的复合终点(1492的[5%] [1492]与2504的[174] [7%];危险比[HR] 0·72、95) %CI 0·57-0·90,p = 0·0047),以及包括肢体截肢在内的严重肢体不良事件(32 [1%] vs 60 [2%]; HR 0·54 95%CI 0·35-0 ·82,p = 0·0037)。利伐沙班5 mg每天两次与单独使用阿司匹林相比,并未显着降低复合终点(2474的149 [6%]比2504的174 [7%]; HR 0·86,95%CI 0·69-1·08, p = 0·19),但减少了包括主要截肢在内的主要不良肢体事件(40 [2%] vs 60 [2%]; HR 0·67,95%CI 0·45-1·00,p = 0·05 )。中位治疗时间为21个月。与单独使用阿司匹林组相比,利伐沙班加阿司匹林联合使用可增加严重出血(2492的77 [3%]比2504的48 [2%]; HR 1·61,95%CI 1·12-2·31, p = 0·0089),主要是胃肠道。同样,在2474例利伐沙班5毫克患者中,有79例(3%)发生了大出血,而阿司匹林单独治疗组中2504例中有48例(2%)(HR 1·68,95%CI 1·17-2·40; p = 0·0043)。解释与单独使用阿司匹林相比,每天服用两次低剂量利伐沙班加阿司匹林可以减少主要的不良心血管和肢体事件。尽管大出血增加了,但致命或关键器官的出血却没有。这种联合疗法代表了在治疗周围动脉疾病患者方面的重要进展。与单独使用阿斯普林相比,单独使用利伐沙班并没有显着减少主要不良心血管事件,但是减少了主要不良肢体事件并增加了严重出血。资助拜耳公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号