Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2017-11-10 , DOI: 10.1016/s0140-6736(17)32458-3 Stuart J Connolly 1 , John W Eikelboom 1 , Jackie Bosch 2 , Gilles Dagenais 3 , Leanne Dyal 1 , Fernando Lanas 4 , Kaj Metsarinne 5 , Martin O'Donnell 6 , Anthony L Dans 7 , Jong-Won Ha 8 , Alexandr N Parkhomenko 9 , Alvaro A Avezum 10 , Eva Lonn 1 , Liu Lisheng 11 , Christian Torp-Pedersen 12 , Petr Widimsky 13 , Aldo P Maggioni 14 , Camilo Felix 15 , Katalin Keltai 16 , Masatsugu Hori 17 , Khalid Yusoff 18 , Tomasz J Guzik 19 , Deepak L Bhatt 20 , Kelley R H Branch 21 , Nancy Cook Bruns 22 , Scott D Berkowitz 23 , Sonia S Anand 1 , John D Varigos 24 , Keith A A Fox 25 , Salim Yusuf 1 ,
The Lancet ( IF 98.4 ) Pub Date : 2017-11-10 , DOI: 10.1016/s0140-6736(17)32458-3 Stuart J Connolly 1 , John W Eikelboom 1 , Jackie Bosch 2 , Gilles Dagenais 3 , Leanne Dyal 1 , Fernando Lanas 4 , Kaj Metsarinne 5 , Martin O'Donnell 6 , Anthony L Dans 7 , Jong-Won Ha 8 , Alexandr N Parkhomenko 9 , Alvaro A Avezum 10 , Eva Lonn 1 , Liu Lisheng 11 , Christian Torp-Pedersen 12 , Petr Widimsky 13 , Aldo P Maggioni 14 , Camilo Felix 15 , Katalin Keltai 16 , Masatsugu Hori 17 , Khalid Yusoff 18 , Tomasz J Guzik 19 , Deepak L Bhatt 20 , Kelley R H Branch 21 , Nancy Cook Bruns 22 , Scott D Berkowitz 23 , Sonia S Anand 1 , John D Varigos 24 , Keith A A Fox 25 , Salim Yusuf 1 ,
Affiliation
|
BACKGROUND
Coronary artery disease is a major cause of morbidity and mortality worldwide, and is a consequence of acute thrombotic events involving activation of platelets and coagulation proteins. Factor Xa inhibitors and aspirin each reduce thrombotic events but have not yet been tested in combination or against each other in patients with stable coronary artery disease.
METHODS
In this multicentre, double-blind, randomised, placebo-controlled, outpatient trial, patients with stable coronary artery disease or peripheral artery disease were recruited at 602 hospitals, clinics, or community centres in 33 countries. This paper reports on patients with coronary artery disease. Eligible patients with coronary artery disease had to have had a myocardial infarction in the past 20 years, multi-vessel coronary artery disease, history of stable or unstable angina, previous multi-vessel percutaneous coronary intervention, or previous multi-vessel coronary artery bypass graft surgery. After a 30-day run in period, patients were randomly assigned (1:1:1) to receive rivaroxaban (2·5 mg orally twice a day) plus aspirin (100 mg once a day), rivaroxaban alone (5 mg orally twice a day), or aspirin alone (100 mg orally once a day). Randomisation was computer generated. Each treatment group was double dummy, and the patients, investigators, and central study staff were masked to treatment allocation. The primary outcome of the COMPASS trial was the occurrence of myocardial infarction, stroke, or cardiovascular death. This trial is registered with ClinicalTrials.gov, number NCT01776424, and is closed to new participants.
FINDINGS
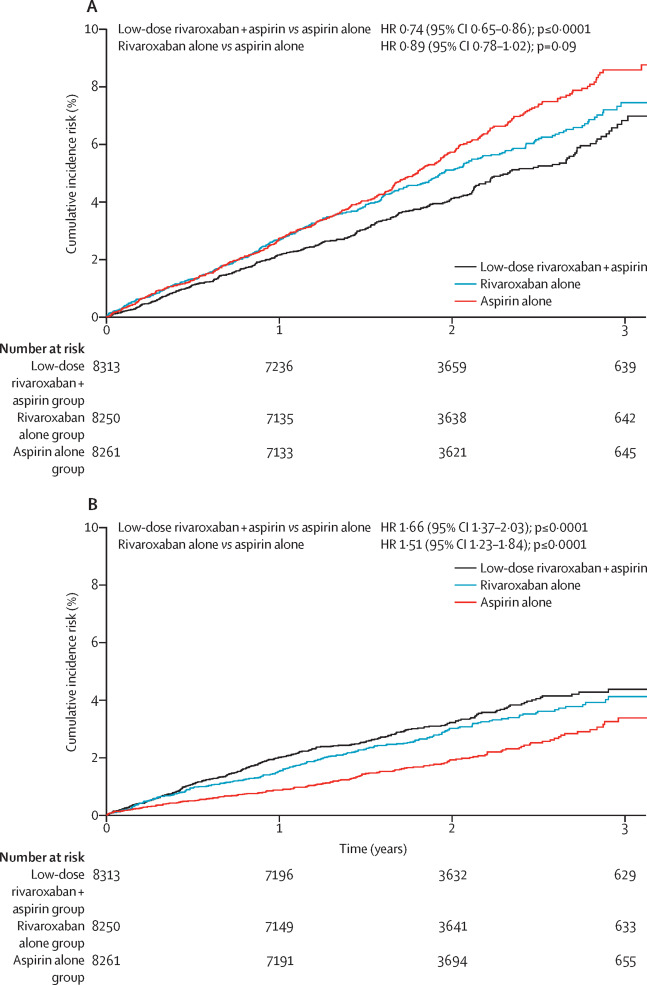
Between March 12, 2013, and May 10, 2016, 27 395 patients were enrolled to the COMPASS trial, of whom 24 824 patients had stable coronary artery disease from 558 centres. The combination of rivaroxaban plus aspirin reduced the primary outcome more than aspirin alone (347 [4%] of 8313 vs 460 [6%] of 8261; hazard ratio [HR] 0·74, 95% CI 0·65-0·86, p<0·0001). By comparison, treatment with rivaroxaban alone did not significantly improve the primary outcome when compared with treatment with aspirin alone (411 [5%] of 8250 vs 460 [6%] of 8261; HR 0·89, 95% CI 0·78-1·02, p=0·094). Combined rivaroxaban plus aspirin treatment resulted in more major bleeds than treatment with aspirin alone (263 [3%] of 8313 vs 158 [2%] of 8261; HR 1·66, 95% CI 1·37-2·03, p<0·0001), and similarly, more bleeds were seen in the rivaroxaban alone group than in the aspirin alone group (236 [3%] of 8250 vs 158 [2%] of 8261; HR 1·51, 95% CI 1·23-1·84, p<0·0001). The most common site of major bleeding was gastrointestinal, occurring in 130 [2%] patients who received combined rivaroxaban plus aspirin, in 84 [1%] patients who received rivaroxaban alone, and in 61 [1%] patients who received aspirin alone. Rivaroxaban plus aspirin reduced mortality when compared with aspirin alone (262 [3%] of 8313 vs 339 [4%] of 8261; HR 0·77, 95% CI 0·65-0·90, p=0·0012).
INTERPRETATION
In patients with stable coronary artery disease, addition of rivaroxaban to aspirin lowered major vascular events, but increased major bleeding. There was no significant increase in intracranial bleeding or other critical organ bleeding. There was also a significant net benefit in favour of rivaroxaban plus aspirin and deaths were reduced by 23%. Thus, addition of rivaroxaban to aspirin has the potential to substantially reduce morbidity and mortality from coronary artery disease worldwide.
FUNDING
Bayer AG.
中文翻译:

稳定型冠状动脉疾病患者中使用或不使用阿司匹林的利伐沙班:一项国际,随机,双盲,安慰剂对照试验。
背景技术冠状动脉疾病是全世界发病率和死亡率的主要原因,并且是涉及血小板和凝血蛋白活化的急性血栓形成事件的结果。Xa因子抑制剂和阿司匹林各自可减少血栓形成事件,但尚未对稳定型冠状动脉疾病患者进行联合测试或相互比较。方法在这项多中心,双盲,随机,安慰剂对照的门诊试验中,在33个国家/地区的602家医院,诊所或社区中心招募了稳定冠状动脉疾病或外周动脉疾病的患者。本文报道了冠心病患者。符合条件的冠心病患者在过去20年中必须患有心肌梗塞,多支冠状动脉疾病,稳定或不稳定型心绞痛的病史,先前的多支血管经皮冠状动脉介入治疗或先前的多支冠状动脉搭桥术。经过一段为期30天的运行,患者被随机分配(1:1:1:1)接受利伐沙班(每天两次口服2·5 mg)加阿司匹林(每天一次100 mg),单独利伐沙班一次(口服5 mg两次)一天)或单独服用阿司匹林(每天一次口服100毫克)。随机化是计算机产生的。每个治疗组均为双重假人,患者,研究者和中心研究人员均被掩盖了治疗分配。COMPASS试验的主要结果是发生心肌梗塞,中风或心血管死亡。该试验已在ClinicalTrials.gov上注册,编号为NCT01776424,并且不对新参与者开放。发现在2013年3月12日至2016年5月10日之间,27 395例患者参加了COMPASS试验,其中558个中心的24 824例患有稳定的冠状动脉疾病。利伐沙班加阿司匹林的组合比单用阿司匹林减少的主要结局更明显(8313的347 [4%]比8261的460 [6%];危险比[HR] 0·74,95%CI 0·65-0·86 ,p <0·0001)。相比之下,与单独使用阿司匹林治疗相比,单独使用利伐沙班治疗并未显着改善主要结局(8250的411 [5%]与8261的460 [6%]; HR 0·89,95%CI 0·78- 1·02,p = 0·094)。与单独使用阿司匹林治疗相比,利伐沙班联合阿司匹林治疗导致更大的出血(8313的263 [3%]比8261的158 [2%]; HR 1·66,95%CI 1·37-2·03,p < 0·0001),并且类似地,仅利伐沙班组的出血量高于阿司匹林组(8250的236 [3%]比8261的158 [2%]; HR 1·51,95%CI 1·23-1·84,p < 0·0001)。最主要的大出血部位是胃肠道,发生在接受利伐沙班加阿司匹林联合治疗的130 [2%]患者,仅接受利伐沙班的84 [1%]患者和仅接受阿司匹林的61 [1%]患者中。与单独使用阿司匹林相比,利伐沙班加阿司匹林可降低死亡率(8313的262 [3%]比8261的339 [4%]; HR 0·77,95%CI 0·65-0·90,p = 0·0012)。解释在稳定的冠状动脉疾病患者中,在阿司匹林中加入利伐沙班可减少主要血管事件,但增加主要出血。颅内出血或其他重要器官出血没有明显增加。利伐沙班加阿司匹林也带来了显着的净收益,死亡人数减少了23%。因此,在阿司匹林中加入利伐沙班有可能显着降低全世界冠状动脉疾病的发病率和死亡率。资助拜耳公司。
更新日期:2018-01-19
中文翻译:

稳定型冠状动脉疾病患者中使用或不使用阿司匹林的利伐沙班:一项国际,随机,双盲,安慰剂对照试验。
背景技术冠状动脉疾病是全世界发病率和死亡率的主要原因,并且是涉及血小板和凝血蛋白活化的急性血栓形成事件的结果。Xa因子抑制剂和阿司匹林各自可减少血栓形成事件,但尚未对稳定型冠状动脉疾病患者进行联合测试或相互比较。方法在这项多中心,双盲,随机,安慰剂对照的门诊试验中,在33个国家/地区的602家医院,诊所或社区中心招募了稳定冠状动脉疾病或外周动脉疾病的患者。本文报道了冠心病患者。符合条件的冠心病患者在过去20年中必须患有心肌梗塞,多支冠状动脉疾病,稳定或不稳定型心绞痛的病史,先前的多支血管经皮冠状动脉介入治疗或先前的多支冠状动脉搭桥术。经过一段为期30天的运行,患者被随机分配(1:1:1:1)接受利伐沙班(每天两次口服2·5 mg)加阿司匹林(每天一次100 mg),单独利伐沙班一次(口服5 mg两次)一天)或单独服用阿司匹林(每天一次口服100毫克)。随机化是计算机产生的。每个治疗组均为双重假人,患者,研究者和中心研究人员均被掩盖了治疗分配。COMPASS试验的主要结果是发生心肌梗塞,中风或心血管死亡。该试验已在ClinicalTrials.gov上注册,编号为NCT01776424,并且不对新参与者开放。发现在2013年3月12日至2016年5月10日之间,27 395例患者参加了COMPASS试验,其中558个中心的24 824例患有稳定的冠状动脉疾病。利伐沙班加阿司匹林的组合比单用阿司匹林减少的主要结局更明显(8313的347 [4%]比8261的460 [6%];危险比[HR] 0·74,95%CI 0·65-0·86 ,p <0·0001)。相比之下,与单独使用阿司匹林治疗相比,单独使用利伐沙班治疗并未显着改善主要结局(8250的411 [5%]与8261的460 [6%]; HR 0·89,95%CI 0·78- 1·02,p = 0·094)。与单独使用阿司匹林治疗相比,利伐沙班联合阿司匹林治疗导致更大的出血(8313的263 [3%]比8261的158 [2%]; HR 1·66,95%CI 1·37-2·03,p < 0·0001),并且类似地,仅利伐沙班组的出血量高于阿司匹林组(8250的236 [3%]比8261的158 [2%]; HR 1·51,95%CI 1·23-1·84,p < 0·0001)。最主要的大出血部位是胃肠道,发生在接受利伐沙班加阿司匹林联合治疗的130 [2%]患者,仅接受利伐沙班的84 [1%]患者和仅接受阿司匹林的61 [1%]患者中。与单独使用阿司匹林相比,利伐沙班加阿司匹林可降低死亡率(8313的262 [3%]比8261的339 [4%]; HR 0·77,95%CI 0·65-0·90,p = 0·0012)。解释在稳定的冠状动脉疾病患者中,在阿司匹林中加入利伐沙班可减少主要血管事件,但增加主要出血。颅内出血或其他重要器官出血没有明显增加。利伐沙班加阿司匹林也带来了显着的净收益,死亡人数减少了23%。因此,在阿司匹林中加入利伐沙班有可能显着降低全世界冠状动脉疾病的发病率和死亡率。资助拜耳公司。











































 京公网安备 11010802027423号
京公网安备 11010802027423号