当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Magnetic Separation of Elastin-like Polypeptide Receptors for Enrichment of Cellular and Molecular Targets
Nano Letters ( IF 9.6 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.nanolett.7b04318 Duy Tien Ta 1, 2 , Rosario Vanella 1, 2 , Michael A. Nash 1, 2
Nano Letters ( IF 9.6 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.nanolett.7b04318 Duy Tien Ta 1, 2 , Rosario Vanella 1, 2 , Michael A. Nash 1, 2
Affiliation
|
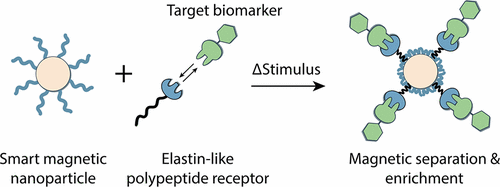
Protein-conjugated magnetic nanoparticles (mNPs) are promising tools for a variety of biomedical applications, from immunoassays and biosensors to theranostics and drug-delivery. In such applications, conjugation of affinity proteins (e.g., antibodies) to the nanoparticle surface many times compromises biological activity and specificity, leading to increased reagent consumption and decreased assay performance. To address this problem, we engineered a biomolecular magnetic separation system that eliminates the need to chemically modify nanoparticles with the capture biomolecules or synthetic polymers of any kind. The system consists of (i) thermoresponsive magnetic iron oxide nanoparticles displaying poly(N-isopropylacrylamide) (pNIPAm), and (ii) an elastin-like polypeptide (ELP) fused with the affinity protein Cohesin (Coh). Proper design of pNIPAm-mNPs and ELP-Coh allowed for efficient cross-aggregation of the two distinct nanoparticle types under collapsing stimuli, which enabled magnetic separation of ELP-Coh aggregates bound to target Dockerin (Doc) molecules. Selective resolubilization of the ELP-Coh/Doc complexes was achieved under intermediate conditions under which only the pNIPAm-mNPs remained aggregated. We show that ELP-Coh is capable of magnetically separating and purifying nanomolar quantities of Doc as well as eukaryotic whole cells displaying the complementary Doc domain from diluted human plasma. This modular system provides magnetic enrichment and purification of captured molecular targets and eliminates the requirement of biofunctionalization of magnetic nanoparticles to achieve bioseparations. Our streamlined and simplified approach is amenable for point-of-use applications and brings the advantages of ELP-fusion proteins to the realm of magnetic particle separation systems.
中文翻译:

磁性分离弹性蛋白样多肽受体的细胞和分子靶标的富集
蛋白质缀合的磁性纳米颗粒(mNP)是从免疫测定和生物传感器到诊断学和药物递送等各种生物医学应用的有前途的工具。在这样的应用中,亲和蛋白(例如抗体)与纳米颗粒表面的结合多次损害了生物活性和特异性,从而导致试剂消耗增加和测定性能下降。为了解决这个问题,我们设计了一种生物分子磁分离系统,无需使用捕获的生物分子或任何种类的合成聚合物对纳米颗粒进行化学修饰。该系统由(i)表现出聚(N-异丙基丙烯酰胺)(pNIPAm),以及(ii)与亲和蛋白Cohesin(Coh)融合的弹性蛋白样多肽(ELP)。正确设计pNIPAm-mNPs和ELP-Coh,可以在折叠刺激下有效交叉聚集两种不同的纳米颗粒,从而实现与目标Dockerin(Doc)分子结合的ELP-Coh聚集体的磁分离。在仅pNIPAm-mNP保持聚集的中间条件下,实现了ELP-Coh / Doc复合物的选择性再溶解。我们显示,ELP-Coh能够从稀释的人血浆中磁分离和纯化纳摩尔浓度的Doc以及展示互补Doc域的真核全细胞。该模块化系统提供了对捕获的分子靶标的磁性富集和纯化,并且消除了对磁性纳米粒子进行生物功能化以实现生物分离的需求。我们简化和简化的方法适用于使用点应用,并将ELP融合蛋白的优势带入了磁性颗粒分离系统领域。
更新日期:2017-11-11
中文翻译:

磁性分离弹性蛋白样多肽受体的细胞和分子靶标的富集
蛋白质缀合的磁性纳米颗粒(mNP)是从免疫测定和生物传感器到诊断学和药物递送等各种生物医学应用的有前途的工具。在这样的应用中,亲和蛋白(例如抗体)与纳米颗粒表面的结合多次损害了生物活性和特异性,从而导致试剂消耗增加和测定性能下降。为了解决这个问题,我们设计了一种生物分子磁分离系统,无需使用捕获的生物分子或任何种类的合成聚合物对纳米颗粒进行化学修饰。该系统由(i)表现出聚(N-异丙基丙烯酰胺)(pNIPAm),以及(ii)与亲和蛋白Cohesin(Coh)融合的弹性蛋白样多肽(ELP)。正确设计pNIPAm-mNPs和ELP-Coh,可以在折叠刺激下有效交叉聚集两种不同的纳米颗粒,从而实现与目标Dockerin(Doc)分子结合的ELP-Coh聚集体的磁分离。在仅pNIPAm-mNP保持聚集的中间条件下,实现了ELP-Coh / Doc复合物的选择性再溶解。我们显示,ELP-Coh能够从稀释的人血浆中磁分离和纯化纳摩尔浓度的Doc以及展示互补Doc域的真核全细胞。该模块化系统提供了对捕获的分子靶标的磁性富集和纯化,并且消除了对磁性纳米粒子进行生物功能化以实现生物分离的需求。我们简化和简化的方法适用于使用点应用,并将ELP融合蛋白的优势带入了磁性颗粒分离系统领域。











































 京公网安备 11010802027423号
京公网安备 11010802027423号