当前位置:
X-MOL 学术
›
Mater. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and properties of open-cage fullerene C60 derivatives: impact of the extended π-conjugation†
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7qm00449d Yoshifumi Hashikawa 1, 2, 3, 4 , Hidefumi Yasui 1, 2, 3, 4 , Kei Kurotobi 1, 2, 3, 4 , Yasujiro Murata 1, 2, 3, 4
Materials Chemistry Frontiers ( IF 6.0 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7qm00449d Yoshifumi Hashikawa 1, 2, 3, 4 , Hidefumi Yasui 1, 2, 3, 4 , Kei Kurotobi 1, 2, 3, 4 , Yasujiro Murata 1, 2, 3, 4
Affiliation
|
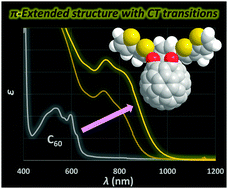
We have developed a method for the synthesis of open-cage fullerene C60 derivatives with extended π-conjugation bearing thienyl groups. By applying this method to an asymmetric diketo derivative, the symmetric form can be obtained without changing the molecular formula. To investigate the structure–property relationship for the asymmetric and symmetric forms, we conducted electrochemical and photophysical measurements. The UV-vis absorption edge was shifted by 210 nm upon changing from the asymmetric to the symmetric form due to the narrower HOMO–LUMO gap, which was also demonstrated by electrochemical analyses. From theoretical calculations, the major contribution of the longest wavelength absorption for the symmetric form is assignable to unusual intramolecular charge transfer transitions whereas π–π* transitions are dominant for the asymmetric form.
中文翻译:

开笼式富勒烯C 60衍生物的合成与性能:扩展π共轭的影响†
我们已经开发了一种开放笼式富勒烯C 60的合成方法具有带有噻吩基的扩展π-共轭的衍生物。通过将该方法应用于不对称二酮衍生物,可以在不改变分子式的情况下获得对称形式。为了研究非对称和对称形式的结构-性质关系,我们进行了电化学和光物理测量。由于较窄的HOMO-LUMO间隙,从不对称形式变为对称形式时,UV-vis吸收边缘移动了210 nm,这也通过电化学分析得到证明。从理论计算来看,对称形式最长的波长吸收的主要贡献可归因于不寻常的分子内电荷转移跃迁,而π-π*跃迁是非对称形式的主导。
更新日期:2017-11-10
中文翻译:

开笼式富勒烯C 60衍生物的合成与性能:扩展π共轭的影响†
我们已经开发了一种开放笼式富勒烯C 60的合成方法具有带有噻吩基的扩展π-共轭的衍生物。通过将该方法应用于不对称二酮衍生物,可以在不改变分子式的情况下获得对称形式。为了研究非对称和对称形式的结构-性质关系,我们进行了电化学和光物理测量。由于较窄的HOMO-LUMO间隙,从不对称形式变为对称形式时,UV-vis吸收边缘移动了210 nm,这也通过电化学分析得到证明。从理论计算来看,对称形式最长的波长吸收的主要贡献可归因于不寻常的分子内电荷转移跃迁,而π-π*跃迁是非对称形式的主导。











































 京公网安备 11010802027423号
京公网安备 11010802027423号