当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Anion Recognition in Aqueous Media by Cyclopeptides and Other Synthetic Receptors
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.accounts.7b00458 Stefan Kubik 1
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1021/acs.accounts.7b00458 Stefan Kubik 1
Affiliation
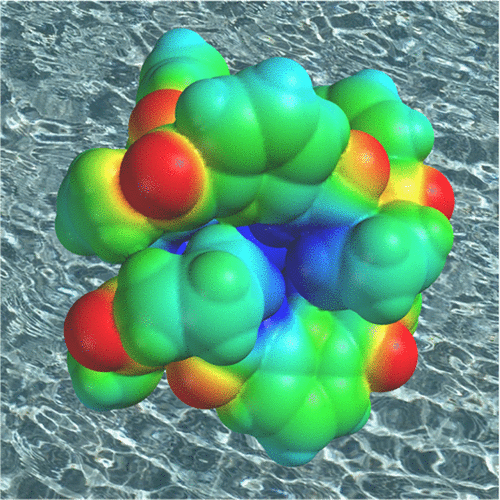
|
Anion receptors often rely on coordinative or multiple ionic interactions to be active in water. In the absence of such strong interactions, anion binding in water can also be efficient, however, as demonstrated by a number of anion receptors developed in recent years. The cyclopeptide-derived receptors comprising an alternating sequence of l-proline and 6-aminopicolinic acid subunits are an example. These cyclopeptides are neutral and, at first sight, can only engage in hydrogen-bond formation with an anionic substrate. Nevertheless, they even interact with strongly solvated sulfate anions in water. The intrinsic anion affinity of these cyclopeptides can be related to structural aspects of their highly preorganized concave binding site, which comprises a wall of hydrophobic proline units arranged around the peptide NH groups at the cavity base. When anions are incorporated into this cavity they can engage in hydrogen-bonding interactions to the NH groups, and complex formation also benefits from cavity dehydration. Formation of 1:1 complexes, in which an anion binds to a single cyclopeptide ring, is associated with only small stability constants, however, whereas significantly more stable complexes are formed if the anion is buried between two cyclopeptide molecules.
中文翻译:

环肽和其他合成受体在水介质中对阴离子的识别
阴离子受体通常依靠配位或多种离子相互作用在水中具有活性。然而,在缺乏这种强相互作用的情况下,水中的阴离子结合也是有效的,正如近年来开发的许多阴离子受体所证明的那样。环肽衍生的受体,其包括的交替序列升-脯氨酸和6-氨基吡啶甲酸亚基是一个例子。这些环肽是中性的,乍看之下只能与阴离子底物形成氢键形成。然而,它们甚至与水中的强溶剂化硫酸根阴离子发生相互作用。这些环肽的固有阴离子亲和力可能与它们的高度预组织的凹形结合位点的结构方面有关,该结构包括凹腔底部围绕肽NH基团排列的疏水性脯氨酸单元壁。当阴离子掺入该腔中时,它们可以与NH基团发生氢键相互作用,并且复合物的形成也得益于腔体的脱水。阴离子与单个环肽环结合的1:1络合物的形成仅具有很小的稳定性常数。
更新日期:2017-11-10
中文翻译:

环肽和其他合成受体在水介质中对阴离子的识别
阴离子受体通常依靠配位或多种离子相互作用在水中具有活性。然而,在缺乏这种强相互作用的情况下,水中的阴离子结合也是有效的,正如近年来开发的许多阴离子受体所证明的那样。环肽衍生的受体,其包括的交替序列升-脯氨酸和6-氨基吡啶甲酸亚基是一个例子。这些环肽是中性的,乍看之下只能与阴离子底物形成氢键形成。然而,它们甚至与水中的强溶剂化硫酸根阴离子发生相互作用。这些环肽的固有阴离子亲和力可能与它们的高度预组织的凹形结合位点的结构方面有关,该结构包括凹腔底部围绕肽NH基团排列的疏水性脯氨酸单元壁。当阴离子掺入该腔中时,它们可以与NH基团发生氢键相互作用,并且复合物的形成也得益于腔体的脱水。阴离子与单个环肽环结合的1:1络合物的形成仅具有很小的稳定性常数。











































 京公网安备 11010802027423号
京公网安备 11010802027423号