PLOS Medicine ( IF 10.5 ) Pub Date : 2017-10-03 , DOI: 10.1371/journal.pmed.1002396 Tara Gomes , David N. Juurlink , Tony Antoniou , Muhammad M. Mamdani , J. Michael Paterson , Wim van den Brink
|
Background
Prescription opioid use is highly associated with risk of opioid-related death, with 1 of every 550 chronic opioid users dying within approximately 2.5 years of their first opioid prescription. Although gabapentin is widely perceived as safe, drug-induced respiratory depression has been described when gabapentin is used alone or in combination with other medications. Because gabapentin and opioids are both commonly prescribed for pain, the likelihood of co-prescription is high. However, no published studies have examined whether concomitant gabapentin therapy is associated with an increased risk of accidental opioid-related death in patients receiving opioids. The objective of this study was to investigate whether co-prescription of opioids and gabapentin is associated with an increased risk of accidental opioid-related mortality.
Methods and findings
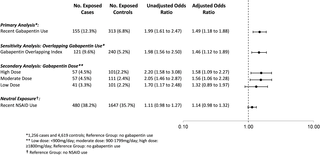
We conducted a population-based nested case–control study among opioid users who were residents of Ontario, Canada, between August 1, 1997, and December 31, 2013, using administrative databases. Cases, defined as opioid users who died of an opioid-related cause, were matched with up to 4 controls who also used opioids on age, sex, year of index date, history of chronic kidney disease, and a disease risk index. After matching, we included 1,256 cases and 4,619 controls. The primary exposure was concomitant gabapentin use in the 120 days preceding the index date. A secondary analysis characterized gabapentin dose as low (<900 mg daily), moderate (900 to 1,799 mg daily), or high (≥1,800 mg daily). A sensitivity analysis examined the effect of concomitant nonsteroidal anti-inflammatory drug (NSAID) use in the preceding 120 days. Overall, 12.3% of cases (155 of 1,256) and 6.8% of controls (313 of 4,619) were prescribed gabapentin in the prior 120 days. After multivariable adjustment, co-prescription of opioids and gabapentin was associated with a significantly increased odds of opioid-related death (odds ratio [OR] 1.99, 95% CI 1.61 to 2.47, p < 0.001; adjusted OR [aOR] 1.49, 95% CI 1.18 to 1.88, p < 0.001) compared to opioid prescription alone. In the dose–response analysis, moderate-dose (OR 2.05, 95% CI 1.46 to 2.87, p < 0.001; aOR 1.56, 95% CI 1.06 to 2.28, p = 0.024) and high-dose (OR 2.20, 95% CI 1.58 to 3.08, p < 0.001; aOR 1.58, 95% CI 1.09 to 2.27, p = 0.015) gabapentin use was associated with a nearly 60% increase in the odds of opioid-related death relative to no concomitant gabapentin use. As expected, we found no significant association between co-prescription of opioids and NSAIDs and opioid-related death (OR 1.11, 95% CI 0.98 to 1.27, p = 0.113; aOR 1.14, 95% CI 0.98 to 1.32, p = 0.083). In an exploratory analysis of patients at risk of combined opioid and gabapentin use, we found that 46.0% (45,173 of 98,288) of gabapentin users in calendar year 2013 received at least 1 concomitant prescription for an opioid. This study was limited to individuals eligible for public drug coverage in Ontario, we were only able to identify prescriptions reimbursed by the government and dispensed from retail pharmacies, and information on indication for gabapentin use was not available. Furthermore, as with all observational studies, confounding due to unmeasured variables is a potential source of bias.
Conclusions
In this study we found that among patients receiving prescription opioids, concomitant treatment with gabapentin was associated with a substantial increase in the risk of opioid-related death. Clinicians should consider carefully whether to continue prescribing this combination of products and, when the combination is deemed necessary, should closely monitor their patients and adjust opioid dose accordingly. Future research should investigate whether a similar interaction exists between pregabalin and opioids.
中文翻译:

加巴喷丁,阿片类药物和与阿片类药物相关的死亡风险:一项基于人群的嵌套病例对照研究
背景
处方阿片类药物的使用与阿片类药物相关的死亡风险高度相关,每550名长期使用阿片类药物的使用者中有1名在其首次使用阿片类药物处方后约2.5年内死亡。尽管加巴喷丁被普遍认为是安全的,但已描述了当单独或与其他药物联合使用加巴喷丁时,药物可引起呼吸抑制。由于加巴喷丁和阿片类药物均通常用于止痛药,因此共同处方的可能性很高。但是,尚无已发表的研究检查加巴喷丁治疗是否与接受阿片类药物的患者中与阿片类药物相关的意外死亡风险增加相关。这项研究的目的是调查阿片类药物和加巴喷丁的共同处方是否与阿片类药物相关的意外死亡风险增加有关。
方法和发现
我们使用行政数据库,在1997年8月1日至2013年12月31日期间,对居住在加拿大安大略省的阿片类药物使用者进行了基于人群的嵌套病例对照研究。病例定义为死于阿片类药物相关原因的阿片类药物使用者,与多达4名对照者相匹配,这些对照组还使用了阿片类药物的年龄,性别,索引日期的年份,慢性肾脏病病史和疾病风险指数。匹配后,我们纳入了1,256个案例和4,619个控件。主要暴露是在索引日期之前的120天内同时使用加巴喷丁。二级分析的特征是加巴喷丁的剂量低(每天<900 mg),中(每天900至1,799 mg)或高(每天≥1,800mg)。敏感性分析检查了前120天内同时使用非甾体抗炎药(NSAID)的效果。总体来说12。在之前的120天内,加巴喷丁的使用率为3%(1,256例中的155例)和6.8%对照(4,619例中的313例)。经过多变量调整后,阿片类药物和加巴喷丁的共同处方与阿片类药物相关的死亡几率显着增加有关(赔率[OR]为1.99,95%CI为1.61至2.47,p < 0.001;与单独的阿片类药物处方相比,校正后的OR [aOR]为1.49,95%CI为1.18至1.88,p < 0.001)。在剂量反应分析中,中等剂量(OR 2.05,95 %CI 1.46至2.87,p < 0.001; aOR 1.56,95%CI 1.06至2.28,p = 0.024)和大剂量(OR 2.20,95%CI) 1.58至3.08,p < 0.001; aOR 1.58,95%CI 1.09至2.27,p = 0.015)加巴喷丁的使用与阿片类药物相关的死亡几率相比无加巴喷丁的使用增加了近60%。如预期的那样,我们发现阿片类药物和NSAID的共同处方与阿片类药物相关的死亡之间没有显着关联(OR 1.11,95 %CI 0.98至1.27,p = 0.113; aOR 1.14,95%CI 0.98至1.32,p =0.083)。在对具有阿片类药物和加巴喷丁联合使用风险的患者进行的探索性分析中,我们发现,2013日历年有46.0%的加巴喷丁使用者(98,288例中的45,173例)至少接受了1剂阿片类药物的同时处方。这项研究仅限于有资格在安大略省获得公共药物保险的个人,我们只能确定政府报销的处方药,并从零售药房中分发,并且尚无加巴喷丁使用适应症的信息。此外,与所有观察性研究一样,由于无法测量的变量造成的混淆是造成偏差的潜在原因。
结论
在这项研究中,我们发现在接受处方阿片类药物的患者中,加巴喷丁的同时治疗与阿片类药物相关死亡的风险大大增加有关。临床医生应仔细考虑是否继续开这种药物组合,并在认为有必要联合使用时,应密切监视患者并相应地调整阿片类药物的剂量。未来的研究应调查普瑞巴林与阿片类药物之间是否存在类似的相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号