PLOS Medicine ( IF 15.8 ) Pub Date : 2017-10-09 , DOI: 10.1371/journal.pmed.1002403 Celia Dechavanne , Sebastien Dechavanne , Ibrahim Sadissou , Adjimon Gatien Lokossou , Fernanda Alvarado , Magalie Dambrun , Kabirou Moutairou , David Courtin , Gregory Nuel , Andre Garcia , Florence Migot-Nabias , Christopher L. King
|
Background
Transplacental transfer of maternal immunoglobulin G (IgG) to the fetus helps to protect against malaria and other infections in infancy. Recent studies have emphasized the important role of malaria-specific IgG3 in malaria immunity, and its transfer may reduce the risk of malaria in infancy. Human IgGs are actively transferred across the placenta by binding the neonatal Fc receptor (FcRn) expressed within the endosomes of the syncytiotrophoblastic membrane. Histidine at position 435 (H435) provides for optimal Fc–IgG binding. In contrast to other IgG subclasses, IgG3 is highly polymorphic and usually contains an arginine at position 435, which reduces its binding affinity to FcRn in vitro. The reduced binding to FcRn is associated with reduced transplacental transfer and reduced half-life of IgG3 in vivo. Some haplotypes of IgG3 have histidine at position 435. This study examines the hypotheses that the IgG3-H435 variant promotes increased transplacental transfer of malaria-specific antibodies and a prolonged IgG3 half-life in infants and that its presence correlates with protection against clinical malaria during infancy.
Methods and findings
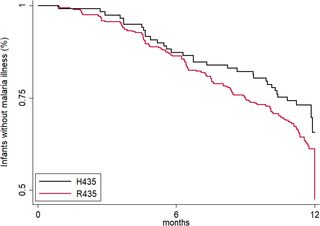
In Benin, 497 mother–infant pairs were included in a longitudinal birth cohort. Both maternal and cord serum samples were assayed for levels of IgG1 and IgG3 specific for MSP119, MSP2 (both allelic families, 3D7 and FC27), MSP3, GLURP (both regions, R0 and R2), and AMA1 antigens of Plasmodium falciparum. Cord:maternal ratios were calculated. The maternal IgG3 gene was sequenced to identify the IgG3-H435 polymorphism. A multivariate logistic regression was used to examine the association between maternal IgG3-H435 polymorphism and transplacental transfer of IgG3, adjusting for hypergammaglobulinemia, maternal malaria, and infant malaria exposure. Twenty-four percent of Beninese women living in an area highly endemic for malaria had the IgG3-H435 allele (377 women homozygous for the IgG3-R435 allele, 117 women heterozygous for the IgG3-R/H alleles, and 3 women homozygous for the IgG3-H435 allele). Women with the IgG3-H435 allele had a 78% (95% CI 17%, 170%, p = 0.007) increased transplacental transfer of GLURP-R2 IgG3 compared to those without the IgG3-H435 allele. Furthermore, in infants born to mothers with the IgG3-H435 variant, a 28% longer IgG3 half-life was noted (95% CI 4%, 59%, p = 0.02) compared to infants born to mothers homozygous for the IgG3-R435 allele. Similar findings were observed for AMA1, MSP2-3D7, MSP3, GLURP-R0, and GLURP-R2 but not for MSP119 and MSP2-FC27. Infants born to women with IgG3-H435 had a 32% lower risk of symptomatic malaria during infancy (incidence rate ratio [IRR] = 0.68 [95% CI 0.51, 0.91], p = 0.01) compared to infants born to mothers homozygous for IgG3-R435. We did not find a lower risk of asymptomatic malaria in infants born to women with or without IgG3-H435. Limitations of the study were the inability to determine (i) the actual amount of IgG3-H435 relative to IgG-R435 in serum samples and (ii) the proportion of malaria-specific IgG produced by infants versus acquired from their mothers.
Conclusions
An arginine-to-histidine replacement at residue 435 in the binding domain of IgG3 to FcRn increases the transplacental transfer and half-life of malaria-specific IgG3 in young infants and is associated with reduced risk of clinical malaria during infancy. The IgG3-H435 allele may be under positive selection, given its relatively high frequency in malaria endemic areas.
中文翻译:

FcRn结合域中的IgG3多态性,疟疾特异性IgG3的经胎盘转移和婴儿期对恶性疟原虫疟疾的保护之间的关联:贝宁的出生队列研究
背景
孕妇的胎盘免疫球蛋白G(IgG)经胎盘转移有助于预防疟疾和婴儿期的其他感染。最近的研究强调了疟疾特异性IgG3在疟疾免疫中的重要作用,其转移可降低婴儿期疟疾的风险。通过结合在合体滋养层膜的内体中表达的新生儿Fc受体(FcRn),人类IgGs主动跨胎盘转移。第435位的组氨酸(H435)可提供最佳的Fc-IgG结合。与其他IgG亚类相比,IgG3具有高度的多态性,通常在435位含有一个精氨酸,从而降低了其与FcRn的结合亲和力。与FcRn的结合减少与体内胎盘转移减少和IgG3的半衰期减少有关。
方法和发现
在贝宁,纵向出生队列中包括497对母婴。分析母体和脐带血清样品中MSP1 19,MSP2(两个等位基因家族,3D7和FC27),MSP3,GLURP(两个区域,R0和R2)和恶性疟原虫的AMA1抗原的特异性IgG1和IgG3水平。计算脐带:母亲比率。对母体IgG3基因进行测序以鉴定IgG3-H435多态性。多元logistic回归用于检查母体IgG3-H435多态性与胎盘IgG3转移之间的关联,并调整高球蛋白血症,母体疟疾和婴儿疟疾暴露水平。生活在疟疾高发地区的贝宁妇女中有24%的人患有IgG3-H435等位基因(其中IgG3-R435等位基因为纯合子的377名妇女,IgG3-R / H等位基因为纯合子的117名妇女,以及IgG3-R / H等位基因为纯合子的3名妇女。 IgG3-H435等位基因)。患有IgG3-H435等位基因的女性有78%(95%CI 17%,170%,p =与没有IgG3-H435等位基因的人相比,GLURP-R2 IgG3的经胎盘转移增加了0.007)。此外,与具有IgG3-R435纯合子的母亲所生的婴儿相比,在具有IgG3-H435变体的母亲所生的婴儿中,IgG3的半衰期要长28%(95%CI 4%,59%,p = 0.02)。等位基因。对于AMA1,MSP2-3D7,MSP3,GLURP-R0和GLURP-R2也观察到了类似的发现,但对于MSP1 19和MSP2-FC27却没有观察到。患有IgG3-H435的妇女所生的婴儿在婴儿期出现症状性疟疾的风险降低了32%(发生率[IRR] = 0.68 [95%CI 0.51,0.91],p =0.01),与纯合母亲的IgG3-R435婴儿相比。对于有或没有IgG3-H435的妇女所生的婴儿,我们没有发现较低的无症状疟疾风险。该研究的局限性在于无法确定(i)血清样品中IgG3-H435相对于IgG-R435的实际量,以及(ii)婴儿产生的与从母亲那里获得的疟疾特异性IgG的比例。
结论
IgG3与FcRn的结合域中第435位残基处的精氨酸-组氨酸置换增加了婴幼儿疟疾特异性IgG3的胎盘转移和半衰期,并降低了婴儿期临床疟疾的风险。鉴于IgG3-H435等位基因在疟疾流行地区的频率相对较高,因此可能处于阳性选择中。



























 京公网安备 11010802027423号
京公网安备 11010802027423号