Journal of Power Sources ( IF 8.1 ) Pub Date : 2017-11-05 , DOI: 10.1016/j.jpowsour.2017.10.086 S. Maletti , A. Sarapulova , A.A. Tsirlin , S. Oswald , F. Fauth , L. Giebeler , N.N. Bramnik , H. Ehrenberg , D. Mikhailova
|
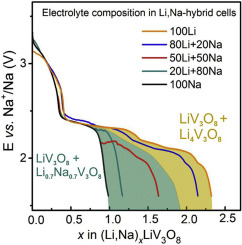
Vanadium(V)-containing oxides show superior intercalation properties for alkaline ions, although the performance of the material strongly depends on its surface morphology. In this work, intercalation activity of LiV3O8, prepared by a conventional solid state synthesis, is demonstrated for the first time in non-aqueous Li,Na-ion hybrid batteries with Na as negative electrode, and different Na/Li ratios in the electrolyte. In the pure Na-ion cell, one Na per formula unit of LiV3O8 can be reversibly inserted at room temperature via a two-step process, while further intercalation leads to gradual amorphisation of the material, with a specific capacity of 190 mAhg−1 after 10 cycles in the potential window of 0.8–3.4 V. Hybrid Li,Na-ion batteries feature simultaneous intercalation of Li+ and Na+ cations into LiV3O8, resulting in the formation of a second phase. Depending on the electrolyte composition, this second phase bears structural similarities either to Li0.7Na0.7V3O8 in Na-rich electrolytes, or to Li4V3O8 in Li-rich electrolytes. The chemical diffusion coefficients of Na+ and Li+ in crystalline LiV3O8 are very close, hence explaining the co-intercalation of these cations. As DFT calculations show, once formed, the Li0.7Na0.7V3O8-type structure favors intercalation of Na+, whereas the LiV3O8-type prefers to accommodate Li+ cations.
中文翻译:

LiV ,Na-离子混合电池中LiV 3 O 8正极的电化学行为
含钒(V)的氧化物对碱性离子表现出优异的嵌入性能,尽管该材料的性能很大程度上取决于其表面形态。在这项工作中,通过传统的固态合成方法制备的LiV 3 O 8的嵌入活性首次在以Na为负极,Na / Li比例不同的非水Li,Na离子混合电池中得到了证明。电解质。在纯Na离子电池中,每个分子单位的LiV 3 O 8可以通过两步过程在室温下可逆地插入一个Na ,而进一步的插入会导致材料逐渐非晶化,比容量为190 mAhg -1在0.8-3.4 V的电势窗口中经过10个循环后。锂,钠离子混合电池的特征是同时将Li +和Na +阳离子嵌入LiV 3 O 8中,导致形成第二相。根据电解质的组成,该第二相在富钠电解质中与Li 0.7 Na 0.7 V 3 O 8或在富锂电解质中与Li 4 V 3 O 8具有结构相似性。Na +和Li +在晶体LiV 3 O 8中的化学扩散系数它们非常接近,因此可以解释这些阳离子的共嵌入。如DFT计算所示,一旦形成Li 0.7 Na 0.7 V 3 O 8型结构,则有利于Na +的嵌入,而LiV 3 O 8型则倾向于容纳Li +阳离子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号