The Lancet Diabetes & Endocrinology ( IF 44.5 ) Pub Date : 2017-09-13 , DOI: 10.1016/s2213-8587(17)30309-1 Rury R Holman , Ruth L Coleman , Juliana C N Chan , Jean-Louis Chiasson , Huimei Feng , Junbo Ge , Hertzel C Gerstein , Richard Gray , Yong Huo , Zhihui Lang , John J McMurray , Lars Rydén , Stefan Schröder , Yihong Sun , Michael J Theodorakis , Michal Tendera , Lynne Tucker , Jaakko Tuomilehto , Yidong Wei , Wenying Yang , Duolao Wang , Dayi Hu , Changyu Pan , Joanne Keenan , Joanne Milton , Zoe Doran , Chris Bray , Jean L Rouleau , Jane Collier , Stuart Pocock , Eberhard Standl , Karl Swedberg , Jianping Weng , Dong Zhao , Mark C Petrie , Eugene Connolly , Pardeep Jhund , Michael MacDonald , Rachel C Myles , Rong Bai , Jing Li , Zhaoping Liu , Zhenyu Liu , Dantao Peng , Qiguang Tong , Chunxue Wang , Xiaowei Yan , Yuqing Zhang , Jingmin Zhou , Naveed Sattar , Miles Fisher , John R Petrie , M Angelyn Bethel , Wen Xu , Sarah Hearn , Anurag Kappai , Shu-Yi Su , Winitha Liyanage , Sanjoy Paul , Emanuela Pozzi , Arne Ring , Rajbir Athwal , Priyanka Batra , Andrea Ferch , Natasha Groves , Irene Kennedy , Olga Nawalaniec , Yash Patel , Rachel Roberts , Victoria Rush , Jayne Starrett , Jennifer Tang , Jing Bi , Zhe Jiang , Hua Wei , Xiaoshuai Wei , Xuan Zhang , Jun Yin , Yu Sun , Rong Hu , Yang Liu , Jianjing Long , Yuefeng Long , Guofang Qiao , Haoyi Qiao , Xiaochun Sun , Yucheng Zhang , Jing Zhou , Bangning Wang , Bin Chen , Lili Deng , Xiaoning Han , Taohong Hu , Qi Hua , Yanming Huo , Hongmei Li , Hongwei Li , Lihua Liu , Juming Lu , Changsheng Ma , Jianjun Peng , Lin Pi , Bin Wang , Guanglin Wei , Ming Yang , Shuyang Zhang , Likun Zhang , Xia Zhao , Yujie Zhou , Libin Shi , Mingsheng Wang , Lirong Wu , Lei Han , Ronghong Liao , Boli Ran , Qiang She , Jiancong Tan , Mei Xia , Chengming Yang , Lianglong Chen , Shangquan Xiong , Ling Yu , Xiaodong Pu , Yan Wang , Qiang Xie , Cibin Chen , Jiyan Chen , Yugang Dong , Zhaohui Wu , Yong Yuan , Wanxing Zhou , Shuxian Zhou , Xiaochao Chen , Chun Wu , Aidong Zhang , Zicheng Li , Shayi Lai , Jin Yang , Jinru Wei , Riyu Kuang , Zilin Zhao , Guoqiang Zhong , Xufen Cao , Yuming Hao , Gang Liu , Dongmei Wang , Hui Fang , Lingjun Kong , Haitao Li , Changqing Wang , Li'na Wang , Xueqi Li , Pingshuan Dong , Shouyan Zhang , Xincan Liu , Yulan Zhao , Hengliang Liu , Ye Gu , Yuhua Liao , Xi Su , Daowen Wang , Hairong Wang , Bo Yang , Ying Guo , Ding'an Ouyang , Tianlun Yang , Yumin Zhang , Yajun Han , Xuefeng Lin , Ruiping Zhao , Ronghai Man , Rongwen Bian , Xu Biao , Buaijiaer Hasimu , Hui Jin , Ping Liu , Jiangyi Yu , Hang Zhang , Chongli Xu , Yan Guo , Ke Lv , Yijia Tao , Xin Xu , Zhenyu Yang , Dongye Li , Chunmei Qi , Guohui Zhang , Xiang Gu , Lang Hong , Ling Hu , Juxiang Li , Ping Yang , Bin Liu , Gang Wang , Hailong Lin , Jun Liu , Shuying Zhang , Ping Han , Yuanzhe Jin , Ling Li , Zhanquan Li , Hong Luan , Mei Song , Li Xue , Yu Hua , Dongwu Liu , Zuyi Yuan , Jixian Ye , Feng Gao , Jinhua Feng , A'li Wang , Shengming Ye , Xiaoyan Li , Guohai Su , Shufang Zhang , Zishan Hou , Wenbin Jiang , Changyong Zhou , Yanping Wang , Wenbo Qi , Xiaomei Bao , Bo Feng , Hui Gong , Shuiming Gu , Mingjun Gu , Xingui Guo , Ben He , Ying Huang , Jinfa Jiang , Yifeng Jiang , Huigen Jin , Yuehua Li , Qiliang Liu , Guoping Lu , Peizhi Miao , Yongwen Qin , Bin Wang , Yuanming Wang , Shiyao Wu , Yawei Xu , Jin Ma , Xiaoping Chen , Xiumin Liu , Jianing Tang , Jingping Wang , Xiaoping Chen , Jianhong Tao , Jun Zhang , Tingjie Zhang , Decai Li , Xinping Du , Tiemin Jiang , Jingna Lin , Chengzhi Lu , Hongjun Ma , Bo Gao , Xukun Guo , Tong Li , Shaoxiong Zheng , Zhongcheng Li , Shuwu Zhao , Qiang Qiu , Kaili Li , Junming Liu , Baopeng Tang , Zhanjun Yuan , Jianhua Zhou , Wenwei Bai , Tao Guo , Ge Zhang , Hong Zhang , Yinglu Hao , Guosheng Fu , Lijiang Tang , Jialun Chen
|
Background
The effect of the α-glucosidase inhibitor acarbose on cardiovascular outcomes in patients with coronary heart disease and impaired glucose tolerance is unknown. We aimed to assess whether acarbose could reduce the frequency of cardiovascular events in Chinese patients with established coronary heart disease and impaired glucose tolerance, and whether the incidence of type 2 diabetes could be reduced.
Methods
The Acarbose Cardiovascular Evaluation (ACE) trial was a randomised, double-blind, placebo-controlled, phase 4 trial, with patients recruited from 176 hospital outpatient clinics in China. Chinese patients with coronary heart disease and impaired glucose tolerance were randomly assigned (1:1), in blocks by site, by a centralised computer system to receive oral acarbose (50 mg three times a day) or matched placebo, which was added to standardised cardiovascular secondary prevention therapy. All study staff and patients were masked to treatment group allocation. The primary outcome was a five-point composite of cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, hospital admission for unstable angina, and hospital admission for heart failure, analysed in the intention-to-treat population (all participants randomly assigned to treatment who provided written informed consent). The secondary outcomes were a three-point composite outcome (cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke), death from any cause, cardiovascular death, fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, hospital admission for unstable angina, hospital admission for heart failure, development of diabetes, and development of impaired renal function. The safety population comprised all patients who received at least one dose of study medication. This trial is registered with ClinicalTrials.gov, number NCT00829660, and the International Standard Randomised Controlled Trial Number registry, number ISRCTN91899513.
Findings
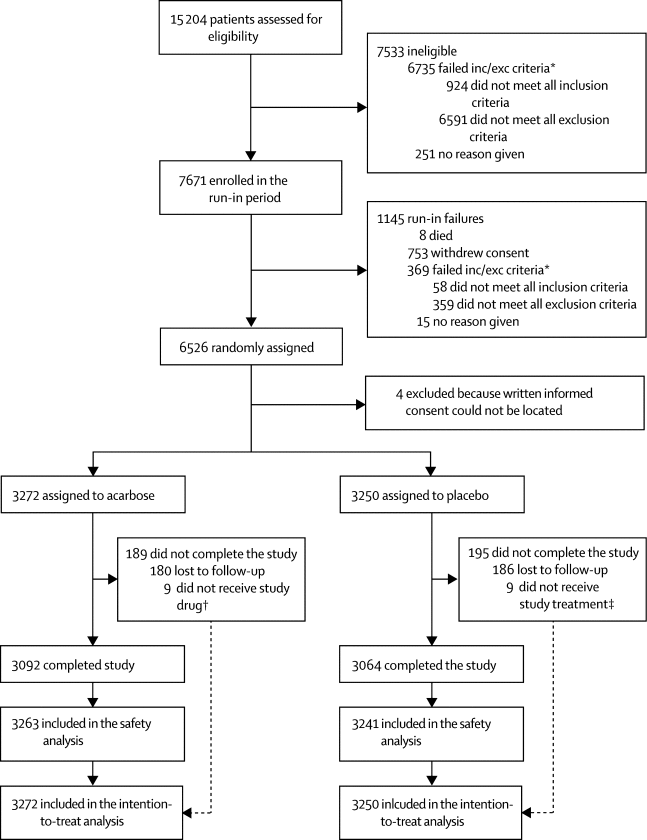
Between March 20, 2009, and Oct 23, 2015, 6522 patients were randomly assigned and included in the intention-to-treat population, 3272 assigned to acarbose and 3250 to placebo. Patients were followed up for a median of 5·0 years (IQR 3·4–6·0) in both groups. The primary five-point composite outcome occurred in 470 (14%; 3·33 per 100 person-years) of 3272 acarbose group participants and in 479 (15%; 3·41 per 100 person-years) of 3250 placebo group participants (hazard ratio 0·98; 95% CI 0·86–1·11, p=0·73). No significant differences were seen between treatment groups for the secondary three-point composite outcome, death from any cause, cardiovascular death, fatal or non-fatal myocardial infarction, fatal or non-fatal stroke, hospital admission for unstable angina, hospital admission for heart failure, or impaired renal function. Diabetes developed less frequently in the acarbose group (436 [13%] of 3272; 3·17 per 100 person-years) compared with the placebo group (513 [16%] of 3250; 3·84 per 100 person-years; rate ratio 0·82, 95% CI 0·71–0·94, p=0·005). Gastrointestinal disorders were the most common adverse event associated with drug discontinuation or dose changes (215 [7%] of 3263 patients in the acarbose group vs 150 [5%] of 3241 in the placebo group [p=0·0007]; safety population). Numbers of non-cardiovascular deaths (71 [2%] of 3272 vs 56 [2%] of 3250, p=0·19) and cancer deaths (ten [<1%] of 3272 vs 12 [<1%] of 3250, p=0·08) did not differ between groups.
Interpretation
In Chinese patients with coronary heart disease and impaired glucose tolerance, acarbose did not reduce the risk of major adverse cardiovascular events, but did reduce the incidence of diabetes.
Funding
Bayer AG.



























 京公网安备 11010802027423号
京公网安备 11010802027423号