The Lancet Infectious Diseases ( IF 36.4 ) Pub Date : 2017-08-29 , DOI: 10.1016/s1473-3099(17)30487-5 Wendelin Moser , Jean T Coulibaly , Said M Ali , Shaali M Ame , Amour K Amour , Richard B Yapi , Marco Albonico , Maxim Puchkov , Jörg Huwyler , Jan Hattendorf , Jennifer Keiser
|
Background
Preventive chemotherapy is the current strategy to control soil-transmitted helminth infections (caused by Ascaris lumbricoides, hookworm, and Trichuris trichiura). But, to improve efficacy and avoid emerging resistance, new drugs are warranted. Tribendimidine has shown good anthelmintic efficacy and is therefore a frontrunner for monotherapy and combination chemotherapy.
Methods
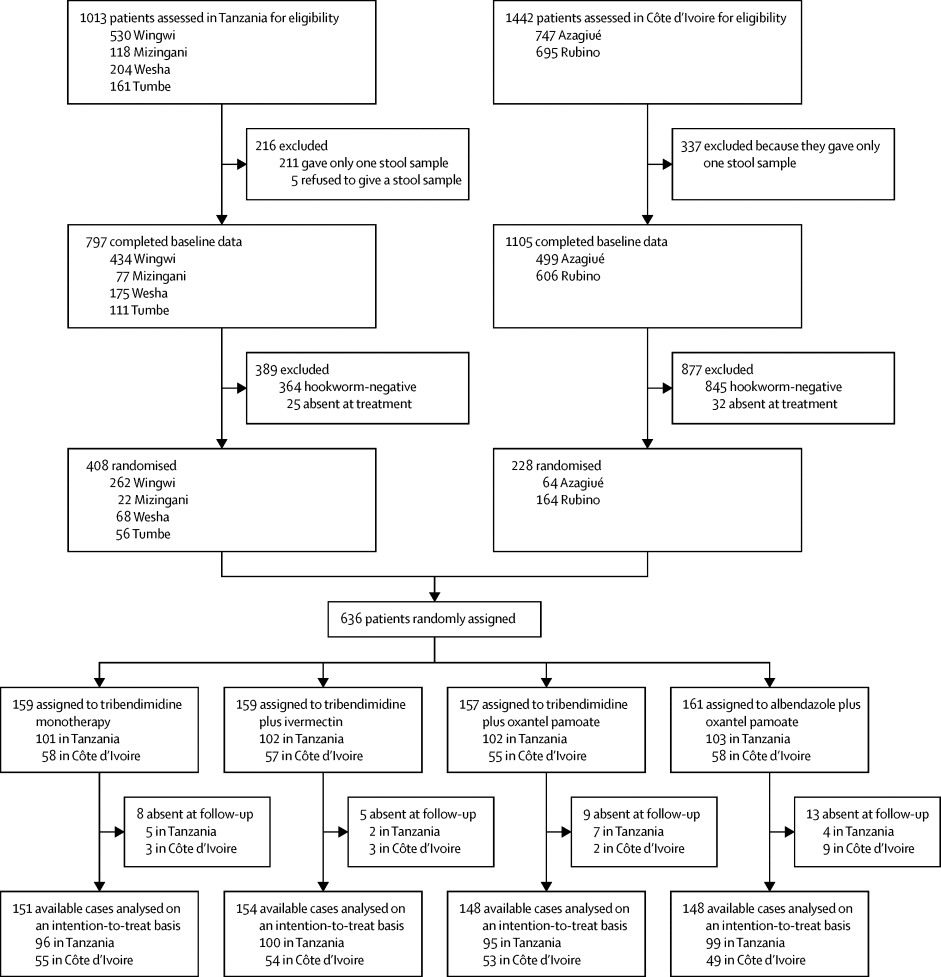
We did a randomised, controlled, single-blinded, non-inferiority trial on Pemba Island, Tanzania, and in Côte d'Ivoire. We recruited adolescents aged 15–18 years from four primary schools on Pemba, and school attendees and non-schoolers from two districts in Côte d'Ivoire. Only hookworm-positive participants were randomly assigned (1:1:1:1) to single, oral doses of tribendimidine 400 mg plus placebo (tribendimidine monotherapy), tribendimidine 400 mg plus ivermectin 200 μg/kg, tribendimidine 400 mg plus oxantel pamoate 25 mg/kg, or albendazole 400 mg plus oxantel pamoate 25 mg/kg. Randomisation was done via a computer-generated list in block sizes of four or eight. Participants were asked to provide two stool samples on 2 consecutive days at baseline and again 14–21 days at follow-up. The primary outcome was the difference in egg-reduction rates (ERRs; ie, the geometric mean reduction) in hookworm egg counts between treatment groups, measured by the Kato-Katz technique. Differences in coadministrated treatment groups were assessed for non-inferiority with a margin of −3% to albendazole plus oxantel pamoate based on the available-case population, analysed by intention to treat. Safety was assessed 3 h and 24 h after treatment. This study is registered with ISRCTN (number 14373201).
Findings
Between July 26, and Dec 23, 2016, we treated 636 hookworm-positive participants, and outcome data were available for 601 participants (151 assigned to tribendimidine monotherapy, 154 to tribendimidine plus ivermectin, 148 to tribendimidine plus oxantel pamoate, and 148 to albendazole plus oxantel pamoate). Tribendimidine plus ivermectin was non-inferior to albendazole plus oxantel pamoate (ERRs 99·5% [95% CI 99·2–99·7] vs 96·0% [93·9–97·4]; difference 3·52 percentage points [2·05–5·65]). Likewise, tribendimidine plus oxantel pamoate was non-inferior to albendazole plus oxantel pamoate (ERRs 96·5% [95% CI 94·9 to 97·6] vs 96·0% [93·9 to 97·4]; difference 0·48 percentage points [–1·61 to 2·88]). 3 h after treatment, headache (n=50 [8%]) and vertigo (n=37 [6%]) were the most widely reported symptoms; 24 h after treatment, 50 (8%) patients reported vertigo and 41 (7%) reported headache. Mainly mild adverse events were reported with peak numbers (n=111 [18%]) at 24 h after treatment. Three participants had moderate adverse events 3 h after treatment: two (<1%) had vertigo and one (<1%) had headache, and two had moderate adverse events 24 h after treatment: one (<1%) had vomiting and one (<1%) had vomiting plus diarrhoea.
Interpretation
Tribendimidine in combination with either ivermectin or oxantel pamoate had a similar, non-inferior efficacy profile as albendazole plus oxantel pamoate, hence tribendimidine will be a useful addition to the depleted anthelmintic drug armamentarium.
Funding
Swiss National Science Foundation.
中文翻译:

Tribendimidine,tribendimidine加伊维菌素,tribendimidine加oxantel pamoate和albendazole加oxantel pamoate在钩虫和伴随土壤传播的蠕虫感染中的功效和安全性:一项随机,对照,单盲,非盲,对照试验
背景
预防性治疗是当前策略,以控制土壤传播的蠕虫感染(由蛔虫,钩虫和鞭虫)。但是,为了提高疗效并避免出现耐药性,必须使用新药。Tribendimidine已显示出良好的驱虫效果,因此是单一疗法和联合化疗的领先者。
方法
我们在坦桑尼亚的奔巴岛和科特迪瓦进行了一项随机,对照,单盲,非自卑性试验。我们从奔巴的四所小学招募了15至18岁的青少年,并从科特迪瓦的两个地区招收了在校生和非在校生。仅钩虫阳性参与者被随机分配(1:1:1:1)单次口服剂量的曲苯二idine 400 mg加安慰剂(曲苯二im单药),曲苯二idine 400 mg加上伊维菌素200μg/ kg,曲苯二idine 400 mg加上黄原酸酯25毫克/千克,或阿苯达唑400毫克加黄原酸黄原酸酯25毫克/千克。通过计算机生成的列表以4或8的块大小进行随机化。要求参与者在基线连续2天提供2个粪便样本,并在随访中再次提供14-21天。主要结果是治疗组之间钩虫卵计数的减蛋率(ERRs,即几何平均减少)的差异,通过加藤-卡茨技术进行了测量。根据可治疗病例分析,评估了联合治疗组的非劣效性差异,其中阿苯达唑加黄原酸羟肟酸酯的非劣性为-3%。治疗后3小时和24小时评估安全性。该研究已在ISRCTN注册(编号14373201)。治疗后3小时和24小时评估安全性。该研究已在ISRCTN注册(编号14373201)。治疗后3小时和24小时评估安全性。该研究已在ISRCTN注册(编号14373201)。
发现
在2016年7月26日至2016年12月23日期间,我们治疗了636例钩虫阳性参与者,并提供了601名参与者的结果数据(151例分配给曲苯咪定单药治疗,154例分配给三苯乙idine加伊维菌素,148例给予三苯乙idine定加氧黄酸氧肟酸酯,148例给阿苯达唑加上黄原酸酯(oxantel pamoate)。Tribendimidine加ivermectin不逊于albendazole加oxantel pamoate(ERRs 99·5%[95%CI 99·2–99·7] vs 96·0%[93·9–97·4];相差3·52百分比点[2·05–5·65])。同样,三苯二咪定加双氧黄ox酸酯也不逊于阿苯达唑加双氧黄pa酸酯(ERRs 96·5%[95%CI 94·9至97·6]与96·0%[93·9至97·4];相差0·48个百分点[–1·61至2·88])。治疗后3小时,头痛(n = 50 [8%])和眩晕(n = 37 [6%])是最广泛报道的症状。治疗后24小时,有50名(8%)患者报告眩晕,有41名(7%)患者报告头痛。在治疗后24小时,主要报道了轻度不良事件,峰值(n = 111 [18%])。三名参与者在治疗3小时后出现中度不良事件:两名(<1%)出现眩晕,一名(<1%)头痛,两名在治疗24小时后出现中度不良事件:一名(<1%)呕吐,一名(<1%)呕吐加腹泻。
解释
Tribendimidine与伊维菌素或oxantel pamoate的组合具有与albendazole加oxantel pamoate相似的,非劣效的功效,因此tribendimidine将是消耗性驱虫药武器库中的有用添加物。
资金
瑞士国家科学基金会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号