The Lancet Oncology ( IF 41.6 ) Pub Date : 2017-10-09 , DOI: 10.1016/s1470-2045(17)30605-8 Tomasz M Beer , Sebastien J Hotte , Fred Saad , Boris Alekseev , Vsevolod Matveev , Aude Fléchon , Gwenaelle Gravis , Florence Joly , Kim N Chi , Zafar Malik , Brent Blumenstein , Patricia S Stewart , Cindy A Jacobs , Karim Fizazi
|
Background
Docetaxel and cabazitaxel improve overall survival compared with mitoxantrone in patients with metastatic castration-resistant prostate cancer. Custirsen (OGX011) is a second generation highly specific antisense oligonucleotide that inhibits the production of clusterin, an antiapoptotic protein that is upregulated in response to chemotherapy and that confers treatment resistance. We aimed to assess whether custirsen in combination with cabazitaxel and prednisone increases overall survival in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel.
Methods
In this randomised, open-label, international, phase 3 trial, men with radiographically documented metastatic castration-resistant prostate cancer that had progressed after docetaxel treatment with a Karnofsky performance status of more than 70% and who were fit for chemotherapy, were recruited from 95 cancer treatment centres in eight countries. Patients were randomly assigned (1:1) centrally using permuted blocks (block size 8) to receive cabazitaxel plus prednisone (cabazitaxel 25 mg/m2 intravenously every 21 days plus oral prednisone 10 mg daily) with or without custirsen (640 mg intravenously on days 1, 8, and 15, plus three previous loading doses) until disease progression, unacceptable toxicity, or the completion of ten treatment cycles. Randomisation was stratified by use of opioids for prostate cancer-related pain at screening, disease progression following first-line docetaxel treatment established by radiographic evidence, and previous treatment with abiraterone or enzalutamide. The co-primary endpoints were overall survival in all randomly assigned patients and in a poor-prognosis subgroup. All analyses were intention to treat with the exception of safety, which was reported for patients who received any assigned treatment. The trial has been completed and the results presented here are the final analysis. This trial is registered with Clinicaltrials.gov, number NCT01578655.
Findings
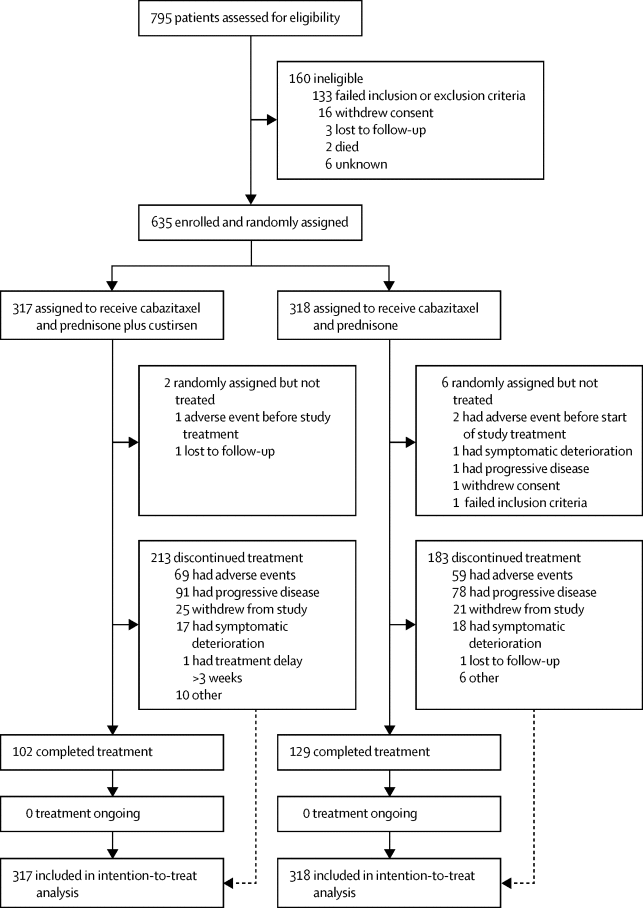
Between Sept 9, 2012, and Sept 29, 2014, 795 patients were screened for enrolment. 635 men were eligible for inclusion and were randomly assigned (n=317 in the cabazitaxel and prednisone plus custirsen group and n=318 in the cabazitaxel and prednisone group). Median follow up was 28·3 months (IQR 24·4–34·5) for the custirsen group and 29·8 months (IQR 25·3–35·2) for the control group. Median overall survival in all randomly assigned patients did not differ between the two groups (14·1 months [95% CI 12·7–15·9] in the curtisen group vs 13·4 months [12·1–14·9] in the control group; hazard ratio [HR] 0·95 [95% CI 0·80–1·12]; log-rank p=0·53). In the poor prognosis subgroup, median overall survival also did not differ between the two treatment groups (11·0 months [95% CI 9·3–13·3] in the custursin group vs 10·9 months [8·2–12·4] in the control group; HR 0·97 [95% CI 0·80–1·21]; two-sided p=0·80). The most frequently reported grade 3 or worse adverse events in the custirsen versus control groups were neutropenia (70 [22%] of 315 vs 61 [20%] of 312), anaemia (68 [22%] vs 49 [16%]), fatigue (23 [7%] vs 18 [6%]), asthenia (16 [5%] vs 8 [3%]), bone pain (16 [5%] vs 5 [2%]), and febrile neutropenia (16 [5%] vs 9 [3%]). Serious adverse events were reported in 155 (49%) versus 132 (42%). 27 patients died within 30 days of treatment in the cabazitaxel and prednisone plus custirsen group, seven of which were deemed to be treatment related, versus 17 in the cabazitaxel and prednisone group, eight of which were deemed to be treatment related. Of the 21 deaths reported, 15 were reported as complications related to study treatment, either chemotherapy (eight and three, respectively) or study drug (none and four, respectively).
Interpretation
We noted no survival benefit in men with metastatic castration-resistant prostate cancer with the addition of custirsen to cabazitaxel and prednisone treatment. Cabazitaxel and prednisone remains the standard of care for patients with metastatic castration-resistant prostate cancer progressing after docetaxel chemotherapy.
Funding
OncoGenex Pharmaceuticals.
中文翻译:

Custirsen(OGX-011)联合卡巴他赛和泼尼松与单独使用卡巴他赛和泼尼松联合治疗已接受多西他赛治疗的转移性去势抵抗性前列腺癌患者(AFFINITY):一项随机,开放标签,国际性的3期临床研究
背景
与米托蒽醌相比,多西他赛和卡巴他赛可改善转移性去势抵抗性前列腺癌患者的总生存率。Custirsen(OGX011)是第二代高度特异性的反义寡核苷酸,可抑制clusterin的产生,簇蛋白是一种抗凋亡蛋白,可响应化学反应而被上调并赋予治疗抵抗力。我们旨在评估在用多西他赛治疗的转移性去势抵抗性前列腺癌患者中,卡西汀与卡巴他赛和强的松联合使用是否能提高总生存率。
方法
在这项随机,开放标签的国际3期试验中,从X线研究中招募了具有放射学记录的转移性去势抵抗性前列腺癌的男性,这些男性在多西他赛治疗后进展,卡诺夫斯基的机能状态超过70%,并且适合化疗。在八个国家/地区设有95个癌症治疗中心。使用置换块(块大小为8)在中心随机分配患者(1:1),以接受卡巴他赛联合泼尼松(卡巴他赛25 mg / m 2)每21天静脉滴注一次,再加口服强的松(每日10 mg),并加或不加Custirsen(在第1、8和15天静脉滴注640 mg,加上之前的三剂剂量),直至疾病进展,不可接受的毒性或完成十个治疗周期。通过在筛选时使用阿片类药物治疗前列腺癌相关疼痛,通过放射线证据确定的一线多西他赛治疗后疾病进展以及先前使用阿比特龙或恩杂鲁胺的治疗,对阿片类药物进行分层。共同主要终点是所有随机分配的患者和预后不良的亚组的总体生存率。除安全性外,所有分析均旨在进行治疗,据报道,该治疗是针对接受任何指定治疗的患者。试验已经完成,此处给出的结果为最终分析。Clinicaltrials.gov,编号NCT01578655。
发现
在2012年9月9日至2014年9月29日之间,对795例患者进行了筛查。635名符合入选条件的男性被随机分配(卡巴他赛和泼尼松加卡迪森组为n = 317,卡巴他赛和泼尼松组为n = 318)。对照组的中位随访时间为28·3个月(IQR 24·4–34·5),对照组为29·8个月(IQR 25·3–35·2)。两组之间所有随机分配的患者的中位总生存期无差异(可迪森组为14·1个月[95%CI 12·7–15·9] vs 13·4个月[12·1-14·9]对照组;危险比[HR] 0·95 [95%CI 0·80-1·12];对数秩p = 0·53)。在预后较差的亚组中,两个治疗组之间的中位总生存期也无差异(库图星组为11·0个月[95%CI 9·3-13·3]对比对照组为10·9个月[8·2–12·4];HR 0·97 [95%CI 0·80-1·21];两侧p = 0·80)。相对于对照组,最常见的3级或更严重的不良事件是嗜中性白血球减少症(315的70 [22%]比312的61 [20%]),贫血(68 [22%]比49 [16%]) ,疲劳(23 [7%]对18 [6%]),乏力(16 [5%]对8 [3%]),骨痛(16 [5%]对5 [2%])和发热性中性粒细胞减少(16 [5%] vs9 [3%])。严重不良事件报告为155(49%),而132(42%)。卡巴他赛和强的松加卡迪森组在治疗后30天内死亡27例患者,其中7例被认为与治疗有关,而卡巴他赛和泼尼松组17例死亡,其中8例被认为与治疗有关。在报告的21例死亡中,有15例被报告为与研究治疗相关的并发症,包括化学疗法(分别为8和3)或研究药物(分别为0和4)。
解释
我们注意到在卡巴他赛和泼尼松治疗中加用卡司汀对转移性去势抵抗性前列腺癌男性没有生存益处。紫杉醇和泼尼松仍然是多西他赛化疗后转移性去势抵抗性前列腺癌患者的标准治疗方法。
资金
OncoGenex药品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号