The Lancet Oncology ( IF 41.6 ) Pub Date : 2017-10-04 , DOI: 10.1016/s1470-2045(17)30624-1 Hussein A Tawbi , Melissa Burgess , Vanessa Bolejack , Brian A Van Tine , Scott M Schuetze , James Hu , Sandra D'Angelo , Steven Attia , Richard F Riedel , Dennis A Priebat , Sujana Movva , Lara E Davis , Scott H Okuno , Damon R Reed , John Crowley , Lisa H Butterfield , Ruth Salazar , Jaime Rodriguez-Canales , Alexander J Lazar , Ignacio I Wistuba , Laurence H Baker , Robert G Maki , Denise Reinke , Shreyaskumar Patel
|
Background
Patients with advanced sarcomas have a poor prognosis and few treatment options that improve overall survival. Chemotherapy and targeted therapies offer short-lived disease control. We assessed pembrolizumab, an anti-PD-1 antibody, for safety and activity in patients with advanced soft-tissue sarcoma or bone sarcoma.
Methods
In this two-cohort, single-arm, open-label, phase 2 study, we enrolled patients with soft-tissue sarcoma or bone sarcoma from 12 academic centres in the USA that were members of the Sarcoma Alliance for Research through Collaboration (SARC). Patients with soft-tissue sarcoma had to be aged 18 years or older to enrol; patients with bone sarcoma could enrol if they were aged 12 years or older. Patients had histological evidence of metastatic or surgically unresectable locally advanced sarcoma, had received up to three previous lines of systemic anticancer therapy, had at least one measurable lesion according to the Response Evaluation Criteria In Solid Tumors version 1.1, and had at least one lesion accessible for biopsy. All patients were treated with 200 mg intravenous pembrolizumab every 3 weeks. The primary endpoint was investigator-assessed objective response. Patients who received at least one dose of pembrolizumab were included in the safety analysis and patients who progressed or reached at least one scan assessment were included in the activity analysis. Accrual is ongoing in some disease cohorts. This trial is registered with ClinicalTrials.gov, number NCT02301039.
Findings
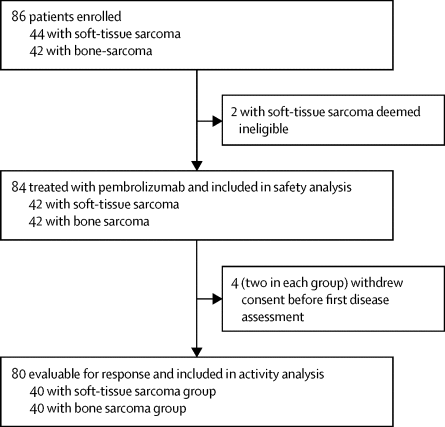
Between March 13, 2015, and Feb 18, 2016, we enrolled 86 patients, 84 of whom received pembrolizumab (42 in each disease cohort) and 80 of whom were evaluable for response (40 in each disease cohort). Median follow-up was 17·8 months (IQR 12·3–19·3). Seven (18%) of 40 patients with soft-tissue sarcoma had an objective response, including four (40%) of ten patients with undifferentiated pleomorphic sarcoma, two (20%) of ten patients with liposarcoma, and one (10%) of ten patients with synovial sarcoma. No patients with leiomyosarcoma (n=10) had an objective response. Two (5%) of 40 patients with bone sarcoma had an objective response, including one (5%) of 22 patients with osteosarcoma and one (20%) of five patients with chondrosarcoma. None of the 13 patients with Ewing's sarcoma had an objective response. The most frequent grade 3 or worse adverse events were anaemia (six [14%]), decreased lymphocyte count (five [12%]), prolonged activated partial thromboplastin time (four [10%]), and decreased platelet count (three [7%]) in the bone sarcoma group, and anaemia, decreased lymphocyte count, and prolonged activated partial thromboplastin time in the soft-tissue sarcoma group (three [7%] each). Nine (11%) patients (five [12%] in the bone sarcoma group and four [10%] in the soft-tissue sarcoma group) had treatment-emergent serious adverse events (SAEs), five of whom had immune-related SAEs, including two with adrenal insufficiency, two with pneumonitis, and one with nephritis.
Interpretation
Pembrolizumab has meaningful clinical activity in patients with undifferentiated pleomorphic sarcoma or dedifferentiated liposarcoma. Enrolment to expanded cohorts of those subtypes is ongoing to confirm and characterise the activity of pembrolizumab.
Funding
Merck, SARC, Sarcoma Foundation of America, QuadW Foundation, Pittsburgh Cure Sarcoma, and Ewan McGregor.
中文翻译:

派姆单抗在晚期软组织肉瘤和骨肉瘤中的应用(SARC028):一项多中心,两队列,单臂,开放标签的2期试验
背景
晚期肉瘤患者预后较差,几乎没有能够提高总体生存率的治疗选择。化学疗法和靶向疗法可提供短暂的疾病控制。我们评估了抗PD-1抗体pembrolizumab在晚期软组织肉瘤或骨肉瘤患者中的安全性和活性。
方法
在这项两队列,单臂,开放标签的2期研究中,我们招募了来自美国12个学术中心的软组织肉瘤或骨肉瘤的患者,这些研究中心是通过协作进行肉瘤研究联盟(SARC)的成员。患有软组织肉瘤的患者必须年满18岁或以上。年龄在12岁或以上的骨肉瘤患者可以入组。患者有组织学证据转移或不能手术切除的局部晚期肉瘤,之前接受过多达三项系统性抗癌治疗,根据《 1.1版实体肿瘤反应评估标准》至少有一个可测量的病灶,并且至少有一个可进入的病灶用于活检。所有患者每3周接受200 mg静脉注射派姆单抗治疗。主要终点是研究者评估的客观反应。安全性分析包括接受至少一剂pembrolizumab的患者,活动性分析包括进行或达到至少一项扫描评估的患者。在某些疾病队列中正在进行应计。该试用版已注册ClinicalTrials.gov,编号NCT02301039。
发现
在2015年3月13日至2016年2月18日之间,我们招募了86位患者,其中84位接受了pembrolizumab(每个疾病队列42个)和80个可评估的反应(每个疾病队列40个)。中位随访时间为17·8个月(IQR 12·3–19·3)。40例软组织肉瘤患者中有7例(18%)有客观反应,包括10例未分化多形性肉瘤患者中的4例(40%),10例脂肪肉瘤患者中的2例(20%)和1例(10%)滑膜肉瘤十例。没有平滑肌肉肉瘤患者(n = 10)有客观反应。40名骨肉瘤患者中有2名(5%)有客观反应,包括22名骨肉瘤患者中的1名(5%)和5名软骨肉瘤患者中的1名(20%)。13例尤因肉瘤患者均未出现客观反应。最常见的3级或更严重的不良事件是贫血(6 [14%]),淋巴细胞计数下降(5 [12%]),活化部分凝血活酶时间延长(4 [10%])和血小板计数下降(3 [骨肉瘤组),贫血,淋巴细胞计数减少和软组织肉瘤组活化部分凝血活酶时间延长(各3例[7%])。九名(11%)患者(骨肉瘤组中五名[12%],软组织肉瘤组中四名[10%])出现了治疗紧急性严重不良事件(SAE),其中五名患有免疫相关的SAE ,其中2例患有肾上腺皮质功能不全,2例患有肺炎和1例患有肾炎。骨肉瘤组血小板计数降低(三[7%]),软组织肉瘤组贫血,淋巴细胞计数降低和活化部分凝血活酶时间延长(各三[7%])。九名(11%)患者(骨肉瘤组中五名[12%],软组织肉瘤组中四名[10%])出现了治疗紧急性严重不良事件(SAE),其中五名患有免疫相关的SAE ,其中2例患有肾上腺皮质功能不全,2例患有肺炎和1例患有肾炎。骨肉瘤组血小板计数降低(三[7%]),软组织肉瘤组贫血,淋巴细胞计数降低和活化部分凝血活酶时间延长(各三[7%])。九名(11%)患者(骨肉瘤组中五名[12%],软组织肉瘤组中四名[10%])出现了治疗紧急性严重不良事件(SAE),其中五名患有免疫相关的SAE ,其中2例患有肾上腺皮质功能不全,2例患有肺炎和1例患有肾炎。
解释
派姆单抗在未分化的多形性肉瘤或未分化的脂肪肉瘤患者中具有有意义的临床活性。正在对这些亚型的扩大队列进行登记,以确认和表征派姆单抗的活性。
资金
默克,SARC,美国肉瘤基金会,QuadW基金会,匹兹堡治愈肉瘤和Ewan McGregor。











































 京公网安备 11010802027423号
京公网安备 11010802027423号