The Lancet Oncology ( IF 41.6 ) Pub Date : 2017-09-25 , DOI: 10.1016/s1470-2045(17)30566-1 Daniel V T Catenacci , Niall C Tebbutt , Irina Davidenko , André M Murad , Salah-Eddin Al-Batran , David H Ilson , Sergei Tjulandin , Evengy Gotovkin , Boguslawa Karaszewska , Igor Bondarenko , Mohamedtaki A Tejani , Anghel A Udrea , Mustapha Tehfe , Ferdinando De Vita , Cheryl Turkington , Rui Tang , Agnes Ang , Yilong Zhang , Tien Hoang , Roger Sidhu , David Cunningham
|
Background
Rilotumumab is a fully human monoclonal antibody that selectively targets the ligand of the MET receptor, hepatocyte growth factor (HGF). We aimed to assess the efficacy, safety, and pharmacokinetics of rilotumumab combined with epirubicin, cisplatin, and capecitabine, and to assess potential biomarkers, in patients with advanced MET-positive gastric or gastro-oesophageal junction adenocarcinoma.
Methods
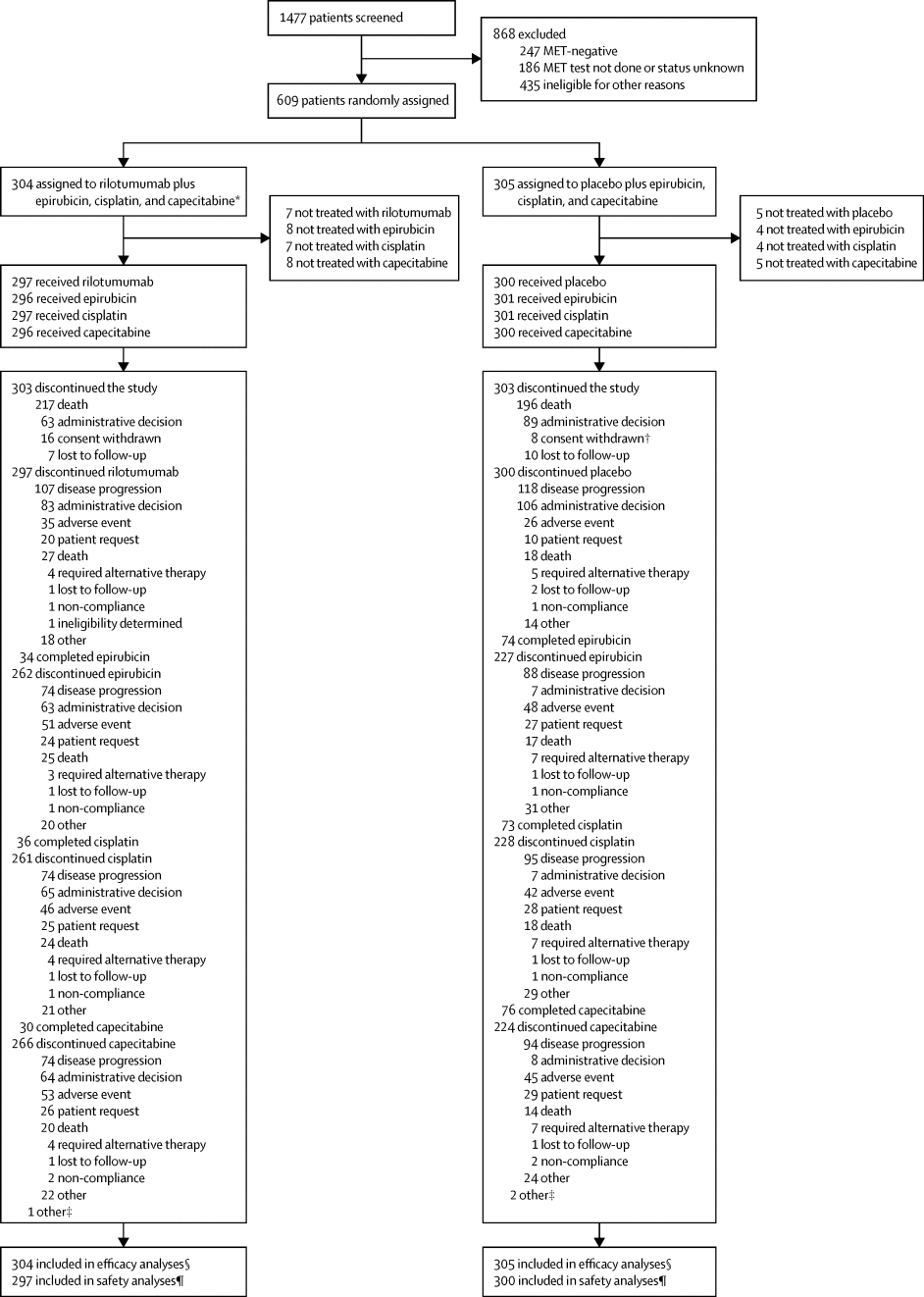
This multicentre, randomised, double-blind, placebo-controlled, phase 3 study was done at 152 centres in 27 countries. We recruited adults (aged ≥18 years) with unresectable locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma, an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, MET-positive tumours (≥25% of tumour cells with membrane staining of ≥1+ staining intensity), and evaluable disease, who had not received previous systemic therapy. Eligible patients were randomly assigned (1:1) via a computerised voice response system to receive rilotumumab 15 mg/kg intravenously or placebo in combination with open-label chemotherapy (epirubicin 50 mg/m2 intravenously; cisplatin 60 mg/m2 intravenously; capecitabine 625 mg/m2 orally twice daily) in 21-day cycles for up to ten cycles. After completion of chemotherapy, patients continued to receive rilotumumab or placebo monotherapy until disease progression, intolerability, withdrawal of consent, or study termination. Randomisation was stratified by disease extent and ECOG performance status. Both patients and physicians were masked to study treatment assignment. The primary endpoint was overall survival, analysed by intention to treat. We report the final analysis. This study is registered with ClinicalTrials.gov, number NCT01697072.
Findings
Between Nov 7, 2012, and Nov 21, 2014, 609 patients were randomly assigned to rilotumumab plus epirubicin, cisplatin, and capecitabine (rilotumumab group; n=304) or placebo plus epirubicin, cisplatin, and capecitabine (placebo group; n=305). Study treatment was stopped early after an independent data monitoring committee found a higher number of deaths in the rilotumumab group than in the placebo group; all patients in the rilotumumab group subsequently discontinued all study treatment. Median follow-up was 7·7 months (IQR 3·6–12·0) for patients in the rilotumumab group and 9·4 months (5·3–13·1) for patients in the placebo group. Median overall survival was 8·8 months (95% CI 7·7–10·2) in the rilotumumab group compared with 10·7 months (9·6–12·4) in the placebo group (stratified hazard ratio 1·34, 95% CI 1·10–1·63; p=0·003). The most common grade 3 or worse adverse events in the rilotumumab and placebo groups were neutropenia (86 [29%] of 298 patients vs 97 [32%] of 299 patients), anaemia (37 [12%] vs 43 [14%]), and fatigue (30 [10%] vs 35 [12%]). The frequency of serious adverse events was similar in the rilotumumab and placebo groups (142 [48%] vs 149 [50%]). More deaths due to adverse events occurred in the rilotumumab group than the placebo group (42 [14%] vs 31 [10%]). In the rilotumumab group, 33 (11%) of 298 patients had fatal adverse events due to disease progression, and nine (3%) had fatal events not due to disease progression. In the placebo group, 23 (8%) of 299 patients had fatal adverse events due to disease progression, and eight (3%) had fatal events not due to disease progression.
Interpretation
Ligand-blocking inhibition of the MET pathway with rilotumumab is not effective in improving clinical outcomes in patients with MET-positive gastric or gastro-oesophageal adenocarcinoma.
Funding
Amgen.
中文翻译:

利洛妥单抗联合表柔比星,顺铂和卡培他滨作为晚期MET阳性胃或胃食管连接癌(RILOMET-1)的一线治疗:一项随机,双盲,安慰剂对照的3期临床试验
背景
Rilotumumab是一种完全人源的单克隆抗体,可选择性靶向MET受体的配体肝细胞生长因子(HGF)。我们的目的是评估利妥单抗联合表柔比星,顺铂和卡培他滨的疗效,安全性和药代动力学,并评估晚期MET阳性胃或胃食管交界性腺癌患者的潜在生物标志物。
方法
这项多中心,随机,双盲,安慰剂对照的3期研究是在27个国家/地区的152个中心进行的。我们招募了成人(≥18岁)不可切除的局部晚期或转移性胃或胃食管交界处腺癌,东部合作肿瘤小组(ECOG)的表现状态为0或1,MET阳性肿瘤(≥25%的肿瘤细胞具有膜染色≥1+染色强度)和可评估的疾病,这些患者以前没有接受过全身性治疗。符合条件的患者通过计算机语音应答系统随机分配(1:1),接受静脉注射利罗妥单抗15 mg / kg或安慰剂联合开放标签化疗(厄比霉素50 mg / m 2静脉内;顺铂60 mg / m 2静脉内;卡培他滨625 mg / m 2每天两次(每天两次)(最多21个周期)。化疗完成后,患者继续接受利洛单抗或安慰剂单一疗法,直至疾病进展,无法忍受,撤回同意或研究终止。随机分组按疾病程度和ECOG表现状态进行分层。患者和医师均被掩盖以研究治疗分配。主要终点是总体生存率,通过治疗意向进行分析。我们报告最终分析。该研究已在ClinicalTrials.gov上注册,编号为NCT01697072。
发现
在2012年11月7日至2014年11月21日之间,随机将609例患者分配至利洛单抗加表柔比星,顺铂和卡培他滨(利洛单抗组; n = 304)或安慰剂加表柔比星,顺铂和卡培他滨(安慰剂组; n = 305) )。在独立数据监测委员会发现利洛妥单抗组的死亡人数高于安慰剂组后,研究治疗被提前终止。利洛单抗组中的所有患者随后均终止了所有研究治疗。利洛单抗组患者的中位随访时间为7·7个月(IQR 3·6-12·0),安慰剂组患者为9·4个月(5·3-13·1)。利洛单抗组中位总生存期为8·8个月(95%CI 7·7-10·2),而安慰剂组为10·7个月(9·6-12·4)(分层风险比为1·34 ,95%CI 1·10-1·63; p = 0·003)。对299例患者中的97个[32%]),贫血(37个[12%]对43个[14%])和疲劳(30个[10%]对35个[12%])。利洛单抗和安慰剂组的严重不良事件发生频率相似(142 [48%] vs 149 [50%])。与安慰剂组相比,利洛妥单抗组因不良事件而死亡的比例更高(42 [14%]对31 [10%])。在利洛单抗组中,298名患者中有33名(11%)因疾病进展而导致致命的不良事件,而9名(3%)因疾病而并非由于疾病进展而导致的致命事件。在安慰剂组中,299名患者中有23名(8%)由于疾病进展而导致致命的不良事件,而8名(3%)具有并非由于疾病进展而导致的致命事件。
解释
利洛单抗对MET途径的配体阻滞抑制作用不能有效改善MET阳性胃或胃食管腺癌患者的临床结局。
资金
安进











































 京公网安备 11010802027423号
京公网安备 11010802027423号