The Lancet ( IF 98.4 ) Pub Date : 2017-09-05 , DOI: 10.1016/s0140-6736(17)31821-4 Warner K Huh , Elmar A Joura , Anna R Giuliano , Ole-Erik Iversen , Rosires Pereira de Andrade , Kevin A Ault , Deborah Bartholomew , Ramon M Cestero , Edison N Fedrizzi , Angelica L Hirschberg , Marie-Hélène Mayrand , Angela Maria Ruiz-Sternberg , Jack T Stapleton , Dorothy J Wiley , Alex Ferenczy , Robert Kurman , Brigitte M Ronnett , Mark H Stoler , Jack Cuzick , Suzanne M Garland , Susanne K Kjaer , Oliver M Bautista , Richard Haupt , Erin Moeller , Michael Ritter , Christine C Roberts , Christine Shields , Alain Luxembourg
|
Background
Primary analyses of a study in young women aged 16–26 years showed efficacy of the nine-valent human papillomavirus (9vHPV; HPV 6, 11, 16, 18, 31, 33, 45, 52, and 58) vaccine against infections and disease related to HPV 31, 33, 45, 52, and 58, and non-inferior HPV 6, 11, 16, and 18 antibody responses when compared with quadrivalent HPV (qHPV; HPV 6, 11, 16, and 18) vaccine. We aimed to report efficacy of the 9vHPV vaccine for up to 6 years following first administration and antibody responses over 5 years.
Methods
We undertook this randomised, double-blind, efficacy, immunogenicity, and safety study of the 9vHPV vaccine study at 105 study sites in 18 countries. Women aged 16–26 years old who were healthy, with no history of abnormal cervical cytology, no previous abnormal cervical biopsy results, and no more than four lifetime sexual partners were randomly assigned (1:1) by central randomisation and block sizes of 2 and 2 to receive three intramuscular injections over 6 months of 9vHPV or qHPV (control) vaccine. All participants, study investigators, and study site personnel, laboratory staff, members of the sponsor's study team, and members of the adjudication pathology panel were masked to vaccination groups. The primary outcomes were incidence of high-grade cervical disease (cervical intraepithelial neoplasia grade 2 or 3, adenocarcinoma in situ, invasive cervical carcinoma), vulvar disease (vulvar intraepithelial neoplasia grade 2/3, vulvar cancer), and vaginal disease (vaginal intraepithelial neoplasia grade 2/3, vaginal cancer) related to HPV 31, 33, 45, 52, and 58 and non-inferiority (excluding a decrease of 1·5 times) of anti-HPV 6, 11, 16, and 18 geometric mean titres (GMT). Tissue samples were adjudicated for histopathology diagnosis and tested for HPV DNA. Serum antibody responses were assessed by competitive Luminex immunoassay. The primary evaluation of efficacy was a superiority analysis in the per-protocol efficacy population, supportive efficacy was analysed in the modified intention-to-treat population, and the primary evaluation of immunogenicity was a non-inferiority analysis. The trial is registered with ClinicalTrials.gov, number NCT00543543.
Findings
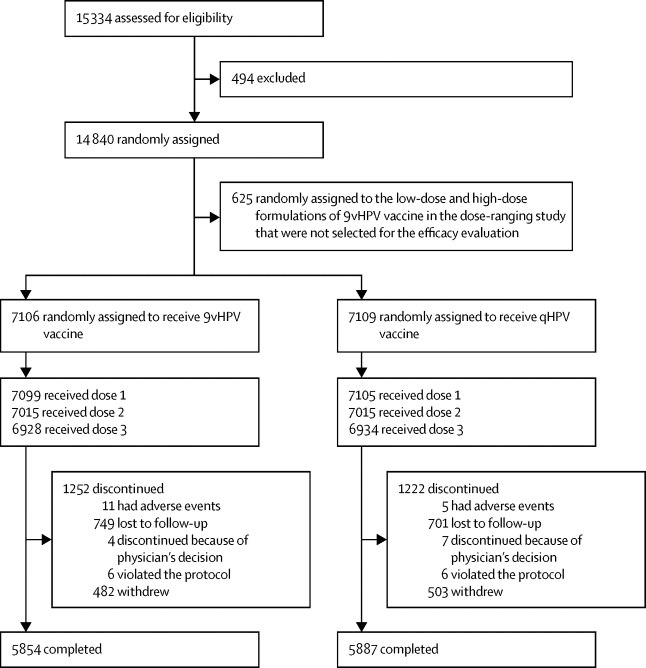
Between Sept 26, 2007, and Dec 18, 2009, we recruited and randomly assigned 14 215 participants to receive 9vHPV (n=7106) or qHPV (n=7109) vaccine. In the per-protocol population, the incidence of high-grade cervical, vulvar and vaginal disease related to HPV 31, 33, 45, 52, and 58 was 0·5 cases per 10 000 person-years in the 9vHPV and 19·0 cases per 10 000 person-years in the qHPV groups, representing 97·4% efficacy (95% CI 85·0–99·9). HPV 6, 11, 16, and 18 GMTs were non-inferior in the 9vHPV versus qHPV group from month 1 to 3 years after vaccination. No clinically meaningful differences in serious adverse events were noted between the study groups. 11 participants died during the study follow-up period (six in the 9vHPV vaccine group and five in the qHPV vaccine group); none of the deaths were considered vaccine-related.
Interpretation
The 9vHPV vaccine prevents infection, cytological abnormalities, high-grade lesions, and cervical procedures related to HPV 31, 33, 45, 52, and 58. Both the 9vHPV vaccine and qHPV vaccine had a similar immunogenicity profile with respect to HPV 6, 11, 16, and 18. Vaccine efficacy was sustained for up to 6 years. The 9vHPV vaccine could potentially provide broader coverage and prevent 90% of cervical cancer cases worldwide.
Funding
Merck & Co, Inc.
中文翻译:

九价人乳头瘤病毒疫苗在16-26岁女性中的最终疗效,免疫原性和安全性分析:一项随机,双盲试验
背景
一项针对16-26岁年轻女性的研究的初步分析显示,九价人乳头瘤病毒(9vHPV; HPV 6、11、16、18、31、33、45、52和58)疫苗对感染和疾病的功效与四价HPV(qHPV; HPV 6、11、16和18)疫苗相比,与HPV 31、33、45、52和58以及非劣等HPV 6、11、16和18抗体反应有关。我们旨在报告9vHPV疫苗在首次给药后长达6年的疗效,并在5年内产生抗体反应。
方法
我们在18个国家/地区的105个研究地点进行了9vHPV疫苗研究的这项随机,双盲,功效,免疫原性和安全性研究。年龄在16-26岁之间的健康女性,无异常子宫颈细胞学病史,以前没有异常的宫颈活检结果,并且通过中央随机分配将随机分配(1:1)的伴侣不超过4个,并且个体大小为2 2个在6个月内接受3次肌内注射9vHPV或qHPV(对照)疫苗。所有参与者,研究调查人员和研究现场人员,实验室工作人员,申办者研究团队的成员以及裁决病理小组的成员都被疫苗接种小组蒙蔽。主要结局是严重宫颈疾病(宫颈上皮内瘤变2或3级,原位腺癌,宫颈癌),与HPV 31、33、45、52和58相关的外阴疾病(外阴上皮内瘤样变2/3级,外阴癌)和阴道病(阴道上皮内瘤样变2/3级,阴道癌) -抗-HPV 6、11、16和18几何平均滴度(GMT)的自卑感(不减少1·5倍)。对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群的优越性分析,改良的意向性治疗人群的支持功效是分析性的,免疫原性的主要评估是非劣效性分析。试用已在 与HPV 31、33、45、52和58和非自卑性(不包括A型)有关的外阴疾病(外阴上皮内瘤样变2/3级,外阴癌)和阴道病(阴道上皮内瘤样变2/3级,阴道癌)抗HPV 6、11、16和18几何平均滴度(GMT)降低1·5倍)。对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在 与HPV 31、33、45、52和58和非自卑性(不包括A型)有关的外阴疾病(外阴上皮内瘤样变2/3级,外阴癌)和阴道病(阴道上皮内瘤样变2/3级,阴道癌)抗HPV 6、11、16和18几何平均滴度(GMT)降低1·5倍)。对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在 阴道癌)与HPV 31、33、45、52和58以及抗HPV 6、11、16和18几何平均滴度(GMT)的非劣效性(不包括降低1·5倍)有关。对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在 阴道癌)与HPV 31、33、45、52和58以及抗HPV 6、11、16和18几何平均滴度(GMT)的非劣效性(不包括降低1·5倍)有关。对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在 对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在 对组织样品进行组织病理学诊断,并检测其HPV DNA。通过竞争性Luminex免疫测定法评估血清抗体应答。疗效的主要评估是按方案有效人群中的优势分析,改良的意向治疗人群中的支持功效是分析性的,免疫原性的主要评估是非劣性分析。试用已在ClinicalTrials.gov,编号NCT00543543。
发现
在2007年9月26日至2009年12月18日期间,我们招募并随机分配了14215名参与者,以接受9vHPV(n = 7106)或qHPV(n = 7109)疫苗。在每个协议人群中,与9vHPV和19·0中的HPV 31、33、45、52和58有关的HPV 31、33、45、52和58的高级宫颈,外阴和阴道疾病的发生率为每10 000人年0·5例。 qHPV组每10 000人年有9例,效率为97·4%(95%CI 85·0–99·9)。在接种疫苗后的1到3年内,在9vHPV与qHPV组中,HPV 6、11、16和18 GMT的表现均不逊色。研究组之间未发现严重不良事件的临床意义差异。在研究随访期间有11名参与者死亡(9vHPV疫苗组中有6名死亡,qHPV疫苗组中有5名死亡);没有死亡病例被认为与疫苗有关。
解释
9vHPV疫苗可预防与HPV 31、33、45、52和58有关的感染,细胞学异常,严重病变和宫颈手术。9vHPV疫苗和qHPV疫苗在HPV 6、11方面具有相似的免疫原性,16和18。疫苗的功效可维持长达6年。9vHPV疫苗可能会提供更广泛的覆盖范围,并预防全球90%的子宫颈癌病例。
资金
默克公司











































 京公网安备 11010802027423号
京公网安备 11010802027423号