当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Trifluoromethylated tertiary homoallylic amines: diastereoselective synthesis and conversion into β-aminoesters, γ- and δ-aminoalcohols, azetidines and pyrrolidines
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7ob02506h Fabienne Grellepois 1, 2, 3, 4, 5 , Abdelkhalek Ben Jamaa 1, 2, 3, 4, 5 , Nathalie Saraiva Rosa 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-10 00:00:00 , DOI: 10.1039/c7ob02506h Fabienne Grellepois 1, 2, 3, 4, 5 , Abdelkhalek Ben Jamaa 1, 2, 3, 4, 5 , Nathalie Saraiva Rosa 1, 2, 3, 4, 5
Affiliation
|
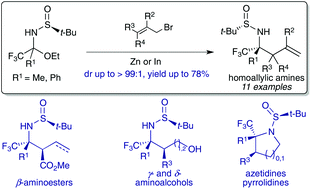
The diastereoselective addition of allyl zinc and allylindium derivatives to α-trifluoromethyl N-tert-butanesulfinyl hemiaminals, bench stable precursors of aryl and alkyl trifluoromethyl ketimines, allows the synthesis of homoallylic amines containing a tetrasubstituted carbon stereocentre bearing a trifluoromethyl group with good diastereoselectivities (up to dr > 99 : 1). This approach was also suitable for accessing chiral homoallylic amines bearing two contiguous stereocenters. The synthetic usefulness of N-tert-butanesulfinyl homoallylamines was illustrated by preparing various trifluoromethylated nitrogen containing bifunctional synthons (aminoesters, aminoalcohols) and small azaheterocycles (azetidines, pyrrolidines).
中文翻译:

α-三氟甲基化叔均烯丙基胺:非对映选择性合成并转化为β-氨基酯,γ-和δ-氨基醇,氮杂环丁烷和吡咯烷
向芳基和烷基三氟甲基酮亚胺的稳定前体α-三氟甲基N-叔丁烷亚磺酰基半缩醛化合物中非对映选择性地添加烯丙基锌和烯丙基化铝,可以合成含有四取代碳立体中心的四烯丙基胺,其具有良好的非对映选择性的三氟甲基博士> 99:1)。该方法也适用于访问带有两个连续立体中心的手性均烯丙基胺。通过制备各种含三氟甲基氮的双功能合成子(氨基酯,氨基醇)和小氮杂杂环(氮杂环丁烷,吡咯烷),可以说明N-叔丁烷亚磺酰基高烯丙基胺的合成用途。
更新日期:2017-11-10
中文翻译:

α-三氟甲基化叔均烯丙基胺:非对映选择性合成并转化为β-氨基酯,γ-和δ-氨基醇,氮杂环丁烷和吡咯烷
向芳基和烷基三氟甲基酮亚胺的稳定前体α-三氟甲基N-叔丁烷亚磺酰基半缩醛化合物中非对映选择性地添加烯丙基锌和烯丙基化铝,可以合成含有四取代碳立体中心的四烯丙基胺,其具有良好的非对映选择性的三氟甲基博士> 99:1)。该方法也适用于访问带有两个连续立体中心的手性均烯丙基胺。通过制备各种含三氟甲基氮的双功能合成子(氨基酯,氨基醇)和小氮杂杂环(氮杂环丁烷,吡咯烷),可以说明N-叔丁烷亚磺酰基高烯丙基胺的合成用途。











































 京公网安备 11010802027423号
京公网安备 11010802027423号