当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphate and phytate adsorption and precipitation on ferrihydrite surfaces
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1039/c7en00705a Xiaoming Wang 1, 2, 3, 4 , Yongfeng Hu 5, 6, 7, 8 , Yadong Tang 9, 10, 11, 12, 13 , Peng Yang 1, 2, 3, 4 , Xionghan Feng 9, 10, 11, 12, 13 , Wenqian Xu 4, 14, 15, 16, 17 , Mengqiang Zhu 1, 2, 3, 4
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2017-09-26 00:00:00 , DOI: 10.1039/c7en00705a Xiaoming Wang 1, 2, 3, 4 , Yongfeng Hu 5, 6, 7, 8 , Yadong Tang 9, 10, 11, 12, 13 , Peng Yang 1, 2, 3, 4 , Xionghan Feng 9, 10, 11, 12, 13 , Wenqian Xu 4, 14, 15, 16, 17 , Mengqiang Zhu 1, 2, 3, 4
Affiliation
|
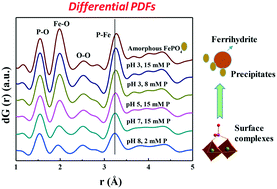
Phosphorous (P) sorption on mineral surfaces largely controls P mobility and bioavailability, hence its pollution potential, but the sorption speciation and mechanism remain poorly understood. We have identified and quantified the speciation of both phosphate and phytate sorbed on ferrihydrite with various P loadings at pH 3–8 using differential atomic pair distribution function (d-PDF) analysis, synchrotron-based X-ray diffraction (XRD), and P and Fe K-edge X-ray absorption near edge structure (XANES) and attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy. With increasing P sorption loading for both phosphate and phytate, the sorption mechanism transits from bidentate-binuclear surface complexation to unidentified ternary complexation and to precipitation of amorphous FePO4 and amorphous Fe-phytate. At a given P sorption loading, phosphate precipitates more readily than phytate. Both phosphate and phytate promote ferrihydrite dissolution with phytate more intensively, but the dissolved FeIII concentration in the bulk solution is low because the majority of the released FeIII precipitate with the anions. Results also show that amorphous FePO4 and amorphous Fe-phytate have similar PO4 local coordination environment. These new insights into the P surface complexation and precipitation, and the ligand-promoted dissolution behavior improve our understanding of P fate in soils, aquatic environment and water treatment systems as mediated by mineral-water interfacial reactions.
中文翻译:

磷酸盐和植酸盐在三水铁矿表面的吸附和沉淀
矿物表面上的磷(P)吸附在很大程度上控制了P的迁移率和生物利用度,因此具有潜在的污染潜力,但对吸附的形态和机理仍知之甚少。我们已经使用差分原子对分布函数(d-PDF)分析,基于同步加速器的X射线衍射(XRD)和P鉴定并量化了在pH为3-8的各种磷负载下吸附在三水铁矿上的磷酸盐和植酸盐的形态。 Fe K边缘X射线吸收近边缘结构(XANES)和衰减全反射傅里叶变换红外(ATR-FTIR)光谱。随着磷酸盐和植酸盐磷吸附量的增加,吸附机理从双齿-双核表面络合物转变为未确定的三元络合物并转变为无定形的FePO 4沉淀。和无定形的植酸铁。在给定的P吸附负荷下,磷酸盐比植酸盐更容易沉淀。磷酸盐和植酸盐都可以更强烈地促进亚铁酸盐与植酸盐的溶解,但是由于大部分释放的Fe III与阴离子一起沉淀,因此本体溶液中溶解的Fe III浓度较低。结果还表明,无定形的FePO 4和无定形的植酸铁具有相似的PO 4局部配位环境。这些对磷表面络合和沉淀以及配体促进的溶解行为的新见解,使我们对矿泉水界面反应介导的土壤,水生环境和水处理系统中磷的命运有了更深入的了解。
更新日期:2017-11-09
中文翻译:

磷酸盐和植酸盐在三水铁矿表面的吸附和沉淀
矿物表面上的磷(P)吸附在很大程度上控制了P的迁移率和生物利用度,因此具有潜在的污染潜力,但对吸附的形态和机理仍知之甚少。我们已经使用差分原子对分布函数(d-PDF)分析,基于同步加速器的X射线衍射(XRD)和P鉴定并量化了在pH为3-8的各种磷负载下吸附在三水铁矿上的磷酸盐和植酸盐的形态。 Fe K边缘X射线吸收近边缘结构(XANES)和衰减全反射傅里叶变换红外(ATR-FTIR)光谱。随着磷酸盐和植酸盐磷吸附量的增加,吸附机理从双齿-双核表面络合物转变为未确定的三元络合物并转变为无定形的FePO 4沉淀。和无定形的植酸铁。在给定的P吸附负荷下,磷酸盐比植酸盐更容易沉淀。磷酸盐和植酸盐都可以更强烈地促进亚铁酸盐与植酸盐的溶解,但是由于大部分释放的Fe III与阴离子一起沉淀,因此本体溶液中溶解的Fe III浓度较低。结果还表明,无定形的FePO 4和无定形的植酸铁具有相似的PO 4局部配位环境。这些对磷表面络合和沉淀以及配体促进的溶解行为的新见解,使我们对矿泉水界面反应介导的土壤,水生环境和水处理系统中磷的命运有了更深入的了解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号