当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the mechanisms of CO2 hydrogenation to methanol on Cu-based catalysts using first-principles multiscale modelling and experiments
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7cy01659j Matej Huš 1, 2, 3, 4 , Drejc Kopač 1, 2, 3, 4 , Neja Strah Štefančič 1, 2, 3, 4 , Damjan Lašič Jurković 1, 2, 3, 4 , Venkata D. B. C. Dasireddy 1, 2, 3, 4 , Blaž Likozar 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7cy01659j Matej Huš 1, 2, 3, 4 , Drejc Kopač 1, 2, 3, 4 , Neja Strah Štefančič 1, 2, 3, 4 , Damjan Lašič Jurković 1, 2, 3, 4 , Venkata D. B. C. Dasireddy 1, 2, 3, 4 , Blaž Likozar 1, 2, 3, 4
Affiliation
|
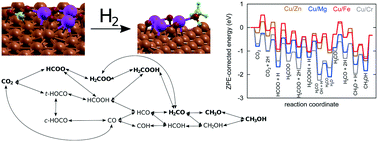
A way to address the challenge of carbon dioxide emissions causing global warming and climate change is the heterogeneous catalytic hydrogenation of CO2. When surplus electrical energy is available, hydrogen may be produced and used for conversion of CO2 into fuels and chemicals. Industrially, a multifunctional metallic copper and zinc oxide catalyst on alumina (CZA) is commonly used. Presently, first-principles multiscale modelling was accomplished for a commercial-like catalyst (Zn3O3/Cu) and three other Cu/metal oxide combinations (Cr3O3/Cu, Fe3O3/Cu, and Mg3O3/Cu), synthesised via co-precipitation, characterised and experimentally tested. Ab initio plane wave density functional theory calculations were performed using different structural models to elucidate the adsorption of intermediates and elementary reaction steps, considering thermodynamics and kinetics. The results were fed into mean-field micro-kinetic expressions to calculate the conversion and selectivity in a continuous flow stirred-tank reactor vessel for various temperatures and pressures. Kinetic Monte Carlo simulations were used to obtain the detailed surface coverage, turnover frequency and catalytic performance. Although no experimental input besides the structure was applied for physico-chemical mechanism description, measurements were in agreement with theoretical predictions. It was shown that the formate species pathway (HCOO → H2COO → H2COOH → H2CO → H3CO) predominates on the examined Cu-based catalysts, although there are variations in the rate determining steps and the most abundant surface intermediate fractions. Whereas Zn3O3/Cu exhibited the highest conversion and a moderate CH3OH product selectivity, the former was lower for Mg3O3/Cu. Interestingly, Cr3O3/Cu was optimal in terms of yield, but with extremely low CH3OH productivity, while Fe3O3/Cu performed poorly overall. Our results highlight the superiority of Zn3O3/Cu, combining the estimations on micro-, meso- and reactor operation scales. Insights beyond traditional activity experiments may be attained and employed in full-scale reactor engineering and optimisation.
中文翻译:

利用第一性原理多尺度建模和实验揭示铜基催化剂上CO 2加氢制甲醇的机理
解决二氧化碳排放引起全球变暖和气候变化的挑战的一种方法是CO 2的非均相催化加氢。当有多余的电能可用时,可能会产生氢气并将其用于将CO 2转化为燃料和化学物质。在工业上,通常使用在氧化铝上的多功能金属铜和锌氧化物催化剂(CZA)。目前,第一原理的多尺度建模是针对商业化催化剂(Zn 3 O 3 / Cu)和其他三种Cu /金属氧化物组合(Cr 3 O 3 / Cu,Fe 3 O 3 / Cu和Mg 3 O)完成的。3/ Cu),通过共沉淀合成,进行表征和实验测试。从头算考虑到热力学和动力学,使用不同的结构模型进行了平面波密度泛函理论计算,以阐明中间体的吸附和基本反应步骤。将结果输入平均场微动力学表达式中,以计算连续流搅拌釜反应器容器中各种温度和压力下的转化率和选择性。动力学蒙特卡洛模拟用于获得详细的表面覆盖率,周转频率和催化性能。尽管除该结构外没有实验输入可用于理化机理描述,但测量结果与理论预测相符。结果表明,甲酸盐类途径(HCOO→H 2 COO→H 2 COOH→H尽管在速率确定步骤和最丰富的表面中间馏分上有变化,但在所研究的铜基催化剂上仍以2 CO→H 3 CO)为主导。Zn 3 O 3 / Cu表现出最高的转化率和适度的CH 3 OH产物选择性,而Mg 3 O 3 / Cu则前者较低。有趣的是,就产量而言,Cr 3 O 3 / Cu是最佳的,但CH 3 OH的生产率极低,而Fe 3 O 3 / Cu的整体性能较差。我们的结果突出了Zn 3 O 3的优越性/ Cu,结合对微型,中观和反应堆运行规模的估计。可以获得超越传统活动实验的见解,并将其用于大规模反应堆工程设计和优化。
更新日期:2017-11-09
中文翻译:

利用第一性原理多尺度建模和实验揭示铜基催化剂上CO 2加氢制甲醇的机理
解决二氧化碳排放引起全球变暖和气候变化的挑战的一种方法是CO 2的非均相催化加氢。当有多余的电能可用时,可能会产生氢气并将其用于将CO 2转化为燃料和化学物质。在工业上,通常使用在氧化铝上的多功能金属铜和锌氧化物催化剂(CZA)。目前,第一原理的多尺度建模是针对商业化催化剂(Zn 3 O 3 / Cu)和其他三种Cu /金属氧化物组合(Cr 3 O 3 / Cu,Fe 3 O 3 / Cu和Mg 3 O)完成的。3/ Cu),通过共沉淀合成,进行表征和实验测试。从头算考虑到热力学和动力学,使用不同的结构模型进行了平面波密度泛函理论计算,以阐明中间体的吸附和基本反应步骤。将结果输入平均场微动力学表达式中,以计算连续流搅拌釜反应器容器中各种温度和压力下的转化率和选择性。动力学蒙特卡洛模拟用于获得详细的表面覆盖率,周转频率和催化性能。尽管除该结构外没有实验输入可用于理化机理描述,但测量结果与理论预测相符。结果表明,甲酸盐类途径(HCOO→H 2 COO→H 2 COOH→H尽管在速率确定步骤和最丰富的表面中间馏分上有变化,但在所研究的铜基催化剂上仍以2 CO→H 3 CO)为主导。Zn 3 O 3 / Cu表现出最高的转化率和适度的CH 3 OH产物选择性,而Mg 3 O 3 / Cu则前者较低。有趣的是,就产量而言,Cr 3 O 3 / Cu是最佳的,但CH 3 OH的生产率极低,而Fe 3 O 3 / Cu的整体性能较差。我们的结果突出了Zn 3 O 3的优越性/ Cu,结合对微型,中观和反应堆运行规模的估计。可以获得超越传统活动实验的见解,并将其用于大规模反应堆工程设计和优化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号