当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Directed Fischer Indolization as an Approach to the Total Syntheses of (+)-Aspidospermidine and (−)-Tabersonine
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.orglett.7b03078 Joo-Young Kim 1 , Chang-Heon Suhl 1 , Joon-Ho Lee 1 , Cheon-Gyu Cho 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.orglett.7b03078 Joo-Young Kim 1 , Chang-Heon Suhl 1 , Joon-Ho Lee 1 , Cheon-Gyu Cho 1
Affiliation
|
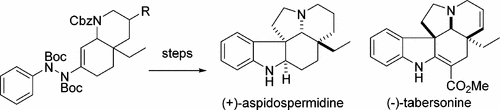
A conceptually new synthetic approach that provides general access to the aspidosperma alkaloids (+)-aspidospermidine and (−)-tabersonine was developed. This method is based on the regioselective indolization of an ene–hydrazide, which was obtained via a base-catalyzed intramolecular aza-Michael reaction, in situ trapping of the resulting enolate, and subsequent C–N coupling with phenyl hydrazide.
中文翻译:

定向费歇尔吲哚化作为(+)-天冬酰胺和(-)-塔布罗宁的总合成方法
开发了一种概念上新颖的合成方法,该方法可提供对蛇形精子生物碱(+)-aspidospermidine和(-)-tabersonine的一般使用途径。该方法基于烯-酰肼的区域选择性吲哚化,这是通过碱催化的分子内氮杂-Michael反应,原位捕获所得的烯醇化物以及随后的CN–N与酰肼的偶联而获得的。
更新日期:2017-11-09
中文翻译:

定向费歇尔吲哚化作为(+)-天冬酰胺和(-)-塔布罗宁的总合成方法
开发了一种概念上新颖的合成方法,该方法可提供对蛇形精子生物碱(+)-aspidospermidine和(-)-tabersonine的一般使用途径。该方法基于烯-酰肼的区域选择性吲哚化,这是通过碱催化的分子内氮杂-Michael反应,原位捕获所得的烯醇化物以及随后的CN–N与酰肼的偶联而获得的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号