当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Co(OAc)2-Catalyzed Trifluoromethylation and C(3)-Selective Arylation of 2-(Propargylamino)pyridines via a 6-Endo-Dig Cyclization
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.orglett.7b02759 Jianjun Li 1 , Yifan Lei 1 , Yang Yu 1 , Cong Qin 1 , Yiwei Fu 1 , Hao Li 1 , Wei Wang 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.orglett.7b02759 Jianjun Li 1 , Yifan Lei 1 , Yang Yu 1 , Cong Qin 1 , Yiwei Fu 1 , Hao Li 1 , Wei Wang 1, 2
Affiliation
|
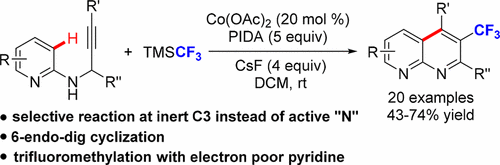
A Co(OAc)2-catalyzed trifluoromethylation and subsequent C(3)-selective arylation of 2-(propargylamino)pyridines has been developed. A new 6-endo-dig cyclization involving an unprecedented C(3) selective arylation of the pyridines instead of a commonly observed 5-exo-dig cyclization with “N” is realized. Moreover, the study presents the first case of the installation of a trifluoromethyl group into electron-deficient azaarenes. The process delivers an efficient cascade approach to new trifluoromethylated 1,8-naphthyridine structures with a broad substrate scope.
中文翻译:

Co(OAc)2催化三氟甲基化和2-(Propargylamino)吡啶通过6 -Endo-Dig环化的C(3)选择性芳基化。
已经开发了Co(OAc)2催化的三氟甲基化和随后的2-(炔丙基氨基)吡啶的C(3)-选择性芳基化。一个新的6 -end-dig环化涉及前所未有的吡啶的C(3)选择性芳基化,而不是通常观察到的带有“ N”的5 -exo-dig环化。此外,该研究提出了在缺乏电子的氮杂芳烃中安装三氟甲基的第一种情况。该方法为具有宽底物范围的新型三氟甲基化1,8-萘啶结构提供了一种有效的级联方法。
更新日期:2017-11-08
中文翻译:

Co(OAc)2催化三氟甲基化和2-(Propargylamino)吡啶通过6 -Endo-Dig环化的C(3)选择性芳基化。
已经开发了Co(OAc)2催化的三氟甲基化和随后的2-(炔丙基氨基)吡啶的C(3)-选择性芳基化。一个新的6 -end-dig环化涉及前所未有的吡啶的C(3)选择性芳基化,而不是通常观察到的带有“ N”的5 -exo-dig环化。此外,该研究提出了在缺乏电子的氮杂芳烃中安装三氟甲基的第一种情况。该方法为具有宽底物范围的新型三氟甲基化1,8-萘啶结构提供了一种有效的级联方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号