JAMA Oncology ( IF 22.5 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.0502 Britt I Drögemöller 1, 2 , Jose G Monzon 3 , Amit P Bhavsar 1, 2 , Adrienne E Borrie 4, 5 , Beth Brooks 6 , Galen E B Wright 2, 7 , Geoffrey Liu 8 , Daniel J Renouf 9 , Christian K Kollmannsberger 9 , Philippe L Bedard 10 , Folefac Aminkeng 2, 7 , Ursula Amstutz 2, 4, 11 , Claudette A Hildebrand 5 , Erandika P Gunaretnam 2, 4 , Carol Critchley 12 , Zhuo Chen 8 , Liam R Brunham 13, 14 , Michael R Hayden 2, 7 , Colin J D Ross 1, 2, 5 , Karen A Gelmon 9 , Bruce C Carleton 4, 5
|
Importance Cisplatin-induced ototoxic effects are an important complication that affects testicular cancer survivors as a consequence of treatment. The identification of genetic variants associated with this adverse drug reaction will further our mechanistic understanding of its development and potentially lead to strategies to prevent ototoxic effects.
Objective To identify the genetic variants associated with cisplatin-induced ototoxic effects in adult testicular cancer patients.
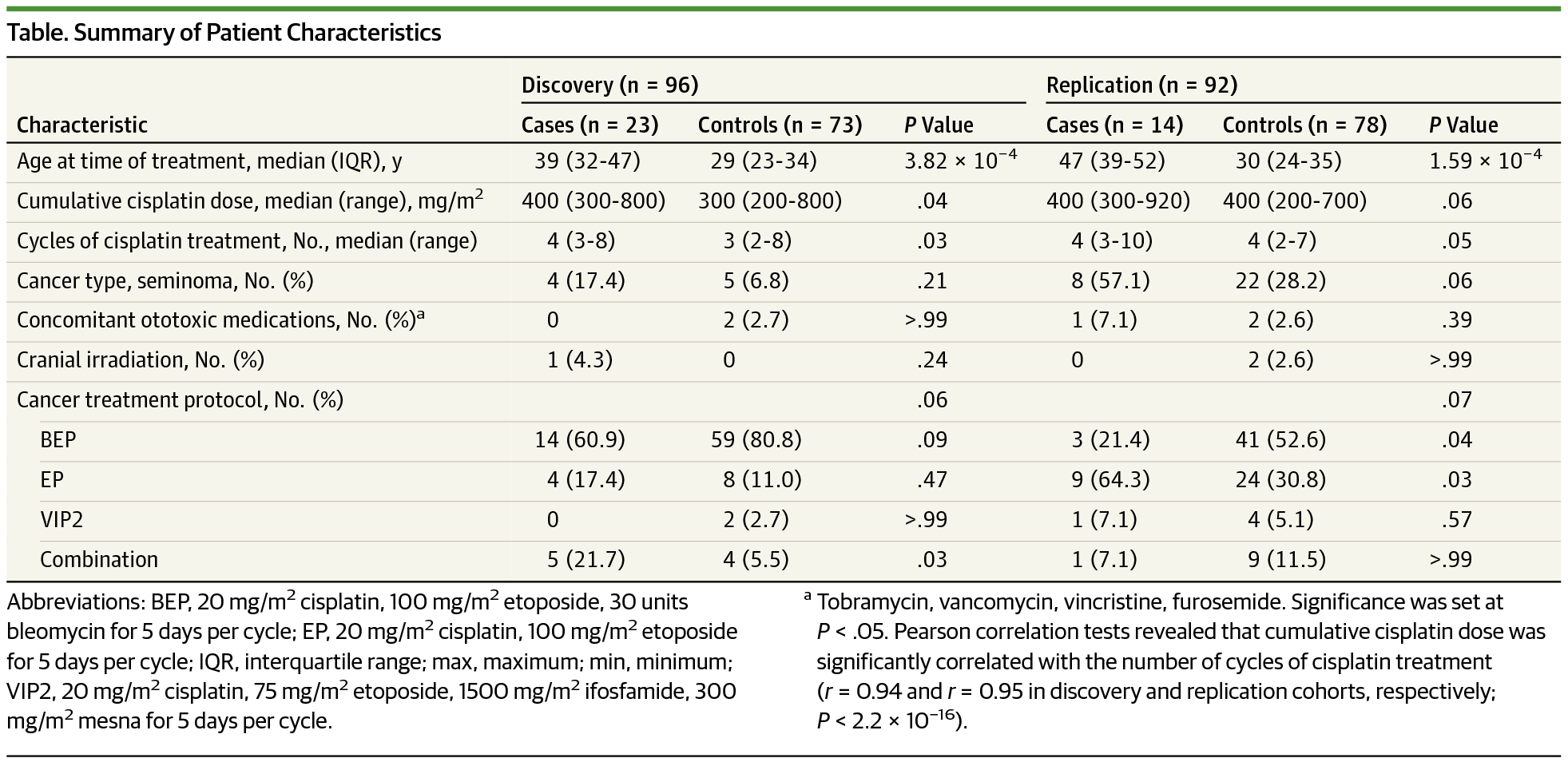
Design, Setting, and Participants This retrospective study was performed by the Canadian Pharmacogenomics Network for Drug Safety using patients recruited from 5 adult oncology treatment centers across Canada. Male patients who were 17 years or older, diagnosed with germ cell testicular cancer, and previously treated with cisplatin-based chemotherapy were recruited from July 2009 to April 2013 using active surveillance methodology. Cisplatin-induced ototoxic effects were independently diagnosed by 2 audiologists. Patients were genotyped for 7907 variants using a custom pharmacogenomic array. Logistic regression was used to identify genetic variants that were significantly associated with ototoxic effects. The validity of these findings was confirmed through independent replication and cell-based functional assays.
Exposures Cisplatin-based chemotherapy.
Main Outcomes and Measures Cisplatin-induced ototoxic effects.
Results After exclusions, 188 patients (median [interquartile range] age, 31 [24-39] years) were enrolled in this study to form the discovery and replication cohorts. Association and fine-mapping analyses identified a protein-coding variant, rs4788863 in SLC16A5, that was associated with protection against cisplatin-induced ototoxic effects in 2 independent cohorts (combined cohort: odds ratio, 0.06; 95% CI, 0.02-0.22; P = 2.17 × 10−7). Functional validation of this transporter gene revealed that in vitro SLC16A5–silencing altered cellular responses to cisplatin treatment, supporting a role for SLC16A5 in the development of cisplatin-induced ototoxic effects. These results were further supported by the literature, which provided confirmatory evidence for the role that SLC16A5 plays in hearing.
Conclusions and Relevance This study has identified a novel association between protein-coding variation in SLC16A5 and cisplatin-induced ototoxic effects. These findings have provided insight into the molecular mechanisms of this adverse drug reaction in adult patients with germ cell testicular cancer. Given that previous studies have shown that cimetidine, an SLC16A5-inhibitor, prevents murine cisplatin-induced ototoxic effects, the findings from this study have important implications for otoprotectant strategies in humans.
中文翻译:

SLC16A5 基因变异与成年睾丸癌患者顺铂诱导的耳毒性作用之间的关联
重要性 顺铂诱导的耳毒性作用是影响睾丸癌幸存者治疗结果的一种重要并发症。与这种药物不良反应相关的遗传变异的鉴定将进一步加深我们对其发展的机制理解,并可能导致预防耳毒性作用的策略。
目的 确定与顺铂诱导的成年睾丸癌患者耳毒性作用相关的遗传变异。
设计、设置和参与者 这项回顾性研究由加拿大药物安全药物基因组学网络使用从加拿大 5 个成人肿瘤治疗中心招募的患者进行。2009 年 7 月至 2013 年 4 月,使用主动监测方法招募了 17 岁或以上、被诊断患有生殖细胞睾丸癌且先前接受过以顺铂为基础的化疗的男性患者。顺铂引起的耳毒性效应由 2 名听力学家独立诊断。使用定制的药物基因组阵列对患者进行了 7907 种变异的基因分型。逻辑回归用于识别与耳毒性效应显着相关的遗传变异。这些发现的有效性通过独立复制和基于细胞的功能测定得到证实。
暴露 以顺铂为基础的化学疗法。
主要结果和措施 顺铂诱导的耳毒性作用。
结果 排除后,188 名患者(中位 [四分位距] 年龄,31 [24-39] 岁)被纳入本研究,形成发现和复制队列。关联和精细定位分析确定了 SLC16A5 中的蛋白质编码变体rs4788863,该变体与 2 个独立队列中的顺铂诱导的耳毒性作用相关(组合队列:优势比,0.06;95% CI,0.02-0.22;P = 2.17 × 10 -7 )。该转运蛋白基因的功能验证表明,体外SLC16A5沉默改变了细胞对顺铂治疗的反应,支持SLC16A5的作用在顺铂诱导的耳毒性作用的发展中。这些结果得到了文献的进一步支持,这些文献为SLC16A5在听力中的作用提供了确证证据。
结论和相关性 本研究确定了SLC16A5中蛋白质编码变异与顺铂诱导的耳毒性作用之间的新关联。这些发现提供了对成年生殖细胞睾丸癌患者这种药物不良反应的分子机制的深入了解。鉴于先前的研究表明,西咪替丁是一种 SLC16A5 抑制剂,可防止小鼠顺铂诱导的耳毒性作用,本研究的结果对人类的耳保护策略具有重要意义。











































 京公网安备 11010802027423号
京公网安备 11010802027423号