JAMA Oncology ( IF 22.5 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.2085 Shuzhen Liu 1 , Bingshu Chen 2 , Samantha Burugu 1 , Samuel Leung 1 , Dongxia Gao 1 , Shakeel Virk 2 , Zuzana Kos 3 , Wendy R Parulekar 2 , Lois Shepherd 2 , Karen A Gelmon 4 , Torsten O Nielsen 1
|
Importance Accumulating evidence indicates that tumor-infiltrating lymphocytes (TILs) are associated with clinical outcomes and may predict the efficacy of chemotherapy and human epidermal growth factor receptor 2 (HER2, encoded by the gene ERBB2)–targeted therapy in patients with HER2-positive breast cancer.
Objective To investigate the role of TILs, particularly cytotoxic CD8+ T cells, in the prediction of outcomes in patients with HER2-positive metastatic breast cancer randomized to an antibody-based (trastuzumab) vs a small molecule–based (lapatinib) anti-HER2 therapy.
Design, Setting, and Participants The Canadian Cancer Trials Group MA.31 phase 3 clinical trial accrued patients from 21 countries and randomized 652 with HER2-positive metastatic breast cancer to receive trastuzumab or lapatinib, in combination with a taxane, from January 17, 2008, through December 1, 2011. Patients had received no prior chemotherapy or HER2-targeted therapy in the metastatic setting. The median follow-up was 21.5 months (interquartile range, 14.3-31.0). The tumor tissue collected for primary diagnosis was used in this ad hoc substudy. Sections were scored for TILs on hematoxylin-eosin (H&E)–stained sections, and immunohistochemical analysis was performed to assess CD8, FOXP3, CD56, and programmed cell death protein 1 (PD-1) expression on stromal (sTILs) and intratumoral TILs. Data were analyzed from July 15, 2015, through July 27, 2016.
Interventions Treatment with trastuzumab or lapatinib in combination with taxane chemotherapy (paclitaxel or docetaxel) for 24 weeks.
Main Outcomes and Measures Prognostic effects of biomarkers were evaluated for progression-free survival by stratified univariate log-rank test with Kaplan-Meier curves and by multivariate Cox proportional hazards regression; predictive effects were examined with a test of interaction between treatment allocation and biomarker classification.
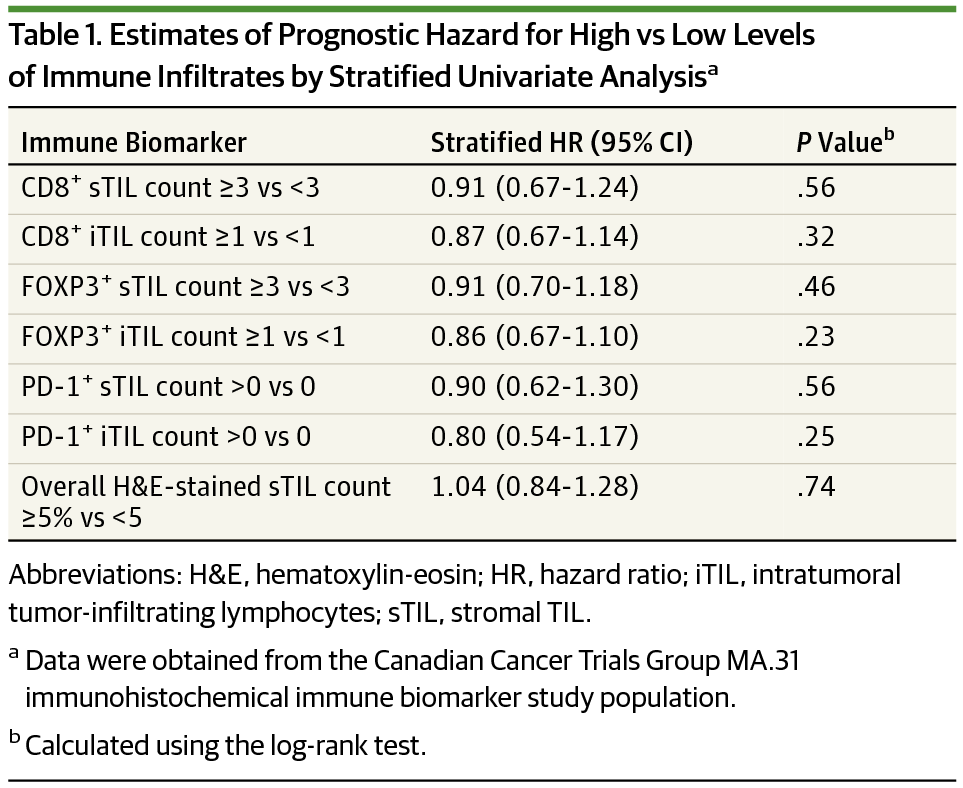
Results Of the 647 treated women (mean [SD] age, 55.0 [10.8] years), 614 had tumor tissue samples scored for H&E sTILs and 427 for CD8 biomarker assessments. Overall H&E sTIL counts of greater than 5% (high) were present in 215 cases (35%) but did not show significant prognostic or predictive effects. Univariate stratified analyses detected a significant predictive effect on risk for progression with lapatinib compared with trastuzumab among patients with low CD8+ sTIL counts (observed hazard ratio, 2.94; 95% CI, 1.40-6.17; P = .003) and among those with high CD8+ sTIL counts (observed hazard ratio, 1.36; 95% CI, 1.05-1.75; P = .02), confirmed in stepwise multivariate analysis (interaction P = .04). Other immunohistochemistry biomarkers were not associated with prognostic or predictive effects.
Conclusions and Relevance In this secondary analysis of a phase 3 randomized clinical trial, a low level of preexisting cytotoxic sTILs predicted the most benefit from an antibody- vs a small molecule–based drug against the same target.
Trial Registration clinicaltrials.gov Identifier: NCT00667251
中文翻译:

细胞毒性肿瘤浸润淋巴细胞在预测转移性 HER2 阳性乳腺癌预后中的作用随机临床试验的二次分析
重要性 越来越多的证据表明肿瘤浸润淋巴细胞 (TIL) 与临床结果相关,并可能预测化疗和人表皮生长因子受体 2 (HER2,由ERBB2基因编码) 靶向治疗对 HER2 阳性乳腺癌患者的疗效癌症。
目的 研究 TILs,尤其是细胞毒性 CD8 + T 细胞,在预测随机接受基于抗体(曲妥珠单抗)和基于小分子(拉帕替尼)抗 HER2 的 HER2 阳性转移性乳腺癌患者预后中的作用治疗。
设计、设置和参与者 加拿大癌症试验组 MA.31 3 期临床试验招募了来自 21 个国家的患者,从 2008 年 1 月 17 日至 2011 年 12 月 1 日,将 652 名 HER2 阳性转移性乳腺癌患者随机分配接受曲妥珠单抗或拉帕替尼联合紫杉烷治疗. 患者在转移性环境中未接受过先前的化疗或 HER2 靶向治疗。中位随访时间为 21.5 个月(四分位距,14.3-31.0)。为初步诊断收集的肿瘤组织用于该特设子研究。对苏木精-伊红 (H&E) 染色切片上的 TIL 切片进行评分,并进行免疫组织化学分析以评估 CD8、FOXP3、CD56 和程序性细胞死亡蛋白 1 (PD-1) 在基质 (sTIL) 和瘤内 TIL 上的表达。数据分析时间为 2015 年 7 月 15 日至 2016 年 7 月 27 日。
干预 曲妥珠单抗或拉帕替尼联合紫杉烷化疗(紫杉醇或多西他赛)治疗 24 周。
主要结果和措施 通过使用 Kaplan-Meier 曲线的分层单变量对数秩检验和多变量 Cox 比例风险回归,评估生物标志物的预后影响的无进展生存期;通过治疗分配和生物标志物分类之间的相互作用测试来检验预测效果。
结果 在 647 名接受治疗的女性(平均 [SD] 年龄,55.0 [10.8] 岁)中,614 名对肿瘤组织样本进行了 H&E sTIL 评分,427 名进行了 CD8 生物标志物评估。215 例 (35%) 的总体 H&E sTIL 计数超过 5%(高),但未显示出显着的预后或预测效果。在 CD8 + sTIL 计数低(观察风险比,2.94;95% CI,1.40-6.17;P = 0.003)和高CD8 + sTIL 计数(观察到的风险比,1.36;95% CI,1.05-1.75;P = .02),在逐步多变量分析中证实(相互作用P = .04)。其他免疫组织化学生物标志物与预后或预测作用无关。
结论和相关性 在这项对 3 期随机临床试验的二次分析中,低水平的预先存在的细胞毒性 sTIL 预测了针对同一靶点的抗体与基于小分子的药物的最大益处。
试验注册 临床试验.gov 标识符:NCT00667251











































 京公网安备 11010802027423号
京公网安备 11010802027423号