JAMA Oncology ( IF 28.4 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.1269 Yoon-Sim Yap 1 , Li-Lian Kwok 2 , Nicholas Syn 3, 4 , Wen Yee Chay 1 , John Whay Kuang Chia 1 , Chee Kian Tham 1 , Nan Soon Wong 1 , Soo Kien Lo 1 , Rebecca Alexandra Dent 1 , Sili Tan 4 , Zuan Yu Mok 4 , King Xin Koh 4 , Han Chong Toh 1 , Wen Hsin Koo 1 , Marie Loh 5, 6 , Raymond Chee Hui Ng 1 , Su Pin Choo 1 , Richie Chuan Teck Soong 4, 7
|
Importance Hand-foot syndrome (HFS) is a common adverse effect of capecitabine treatment.
Objective To compare the incidence and time to onset of grade 2 or greater HFS in patients receiving pyridoxine vs placebo and to identify biomarkers predictive of HFS.
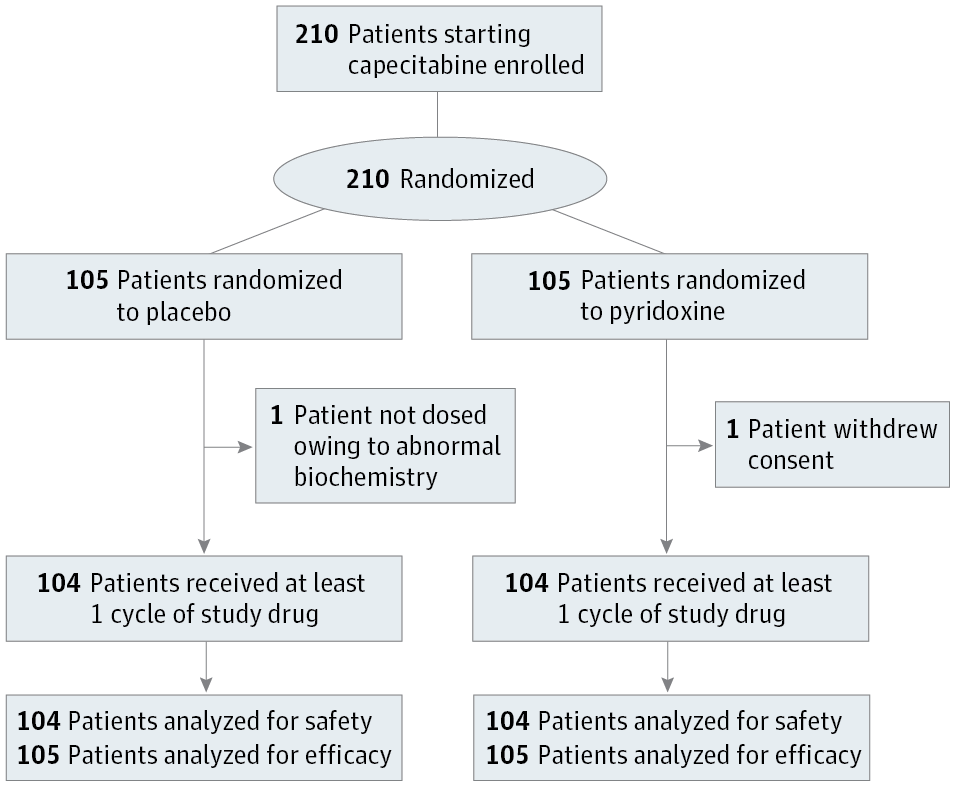
Design, Setting, and Participants This single-center, randomized double-blind, placebo-controlled phase 3 trial conducted at National Cancer Centre Singapore assessed whether oral pyridoxine could prevent the onset of grade 2 or higher HFS in 210 patients scheduled to receive single-agent capecitabine chemotherapy for breast, colorectal, and other cancers.
Interventions Patients were randomized to receive concurrent pyridoxine (200 mg) or placebo daily for a maximum of 8 cycles of capecitabine, with stratification by sex and use in adjuvant or neoadjuvant vs palliative setting. Patients were withdrawn from the study on development of grade 2 or higher HFS or cessation of capecitabine.
Main Outcomes and Measures Primary end point was the incidence of grade 2 or higher HFS in patients receiving pyridoxine. Secondary end points included the time to onset (days) of grade 2 or higher HFS and identification of biomarkers predictive of HFS, including baseline folate and vitamin B12 levels, as well as genetic polymorphisms with genome-wide arrays.
Results In this cohort of 210 patients (median [range] age, 58 [26-82] years; 162 women) grade 2 or higher HFS occurred in 33 patients (31.4%) in the pyridoxine arm vs 39 patients (37.1%) in the placebo arm (P = .38). The median time to onset of grade 2 or higher HFS was not reached in both arms. In univariate analysis, the starting dose of capecitabine (odds ratio [OR], 1.99; 95% CI, 1.32-3.00; P = .001), serum folate levels (OR, 1.27; 95% CI, 1.10-1.47; P = .001), and red blood cell folate levels (OR, 1.25; 95% CI, 1.08-1.44; P = .003) were associated with increased risk of grade 2 or higher HFS. In multivariate analyses, serum folate (OR, 1.30; 95% CI, 1.12-1.52; P < .001) and red blood cell folate (OR, 1.28; 95% CI, 1.10-1.49; P = .001) were the only significant predictors of grade 2 or higher HFS. Grade 2 or higher HFS was associated with 300 DNA variants at genome-wide significance (P < 5 × 10−8), including a novel DPYD variant (rs75267292; P = 1.57 × 10−10), and variants in the MACF1 (rs183324967, P = 4.80 × 10−11; rs148221738, P = 5.73 × 10−10) and SPRY2 (rs117876855, P < 1.01 × 10−8; rs139544515, P = 1.30 × 10−8) genes involved in wound healing.
Conclusions and Relevance Pyridoxine did not significantly prevent or delay the onset of grade 2 or higher HFS. Serum and red blood cell folate levels are independent predictors of HFS.
Trial Registration clinicaltrials.gov Identifier: NCT00486213
中文翻译:

手足综合征的预测因子和吡哆醇预防卡培他滨诱发的手足综合征一项随机临床试验
重要性 手足综合征 (HFS) 是卡培他滨治疗的常见不良反应。
目的 比较接受吡哆醇与安慰剂的患者发生 2 级或更高 HFS 的发生率和时间,并确定预测 HFS 的生物标志物。
设计、设置和参与者 这项在新加坡国家癌症中心进行的单中心、随机双盲、安慰剂对照的 3 期试验评估了口服吡哆醇是否可以预防 210 名计划接受单药治疗的患者发生 2 级或更高的 HFS。用于乳腺癌、结肠直肠癌和其他癌症的药物卡培他滨化学疗法。
干预 患者随机接受每日并发吡哆醇(200 mg)或安慰剂,最多 8 个周期的卡培他滨,按性别分层,用于辅助或新辅助与姑息治疗。患者退出研究发生 2 级或更高 HFS 或停止卡培他滨。
主要结果和措施 主要终点是接受吡哆醇的患者中 2 级或更高 HFS 的发生率。次要终点包括 2 级或更高 HFS 的发病时间(天)和预测 HFS 的生物标志物的鉴定,包括基线叶酸和维生素 B12 水平,以及全基因组阵列的遗传多态性。
结果 在这 210 名患者(中位 [范围] 年龄,58 [26-82] 岁;162 名女性)队列中,吡哆醇组 33 名患者(31.4%)与 39 名患者(37.1%)发生 2 级或更高的 HFS安慰剂组 ( P = .38)。两组均未达到 2 级或更高 HFS 发作的中位时间。在单变量分析中,卡培他滨的起始剂量(优势比 [OR],1.99;95% CI,1.32-3.00;P = .001),血清叶酸水平(OR,1.27;95% CI,1.10-1.47;P = .001)和红细胞叶酸水平(OR,1.25;95% CI,1.08-1.44;P = .003)与 2 级或更高 HFS 的风险增加相关。在多变量分析中,血清叶酸(OR,1.30;95% CI,1.12-1.52;P < .001) 和红细胞叶酸 (OR, 1.28; 95% CI, 1.10-1.49; P = .001) 是 2 级或更高 HFS 的唯一显着预测因子。2 级或更高的 HFS 与全基因组显着性的 300 个 DNA 变体相关 ( P < 5 × 10 -8 ),包括一个新的DPYD变体 ( rs75267292 ; P = 1.57 × 10 -10 ) 和MACF1中的变体( rs183324967 , P = 4.80 × 10 -11 ; rs148221738 , P = 5.73 × 10 -10 ) 和SPRY2 ( rs117876855 , P < 1.01 × 10 -8 ; rs139544515 , P = 1.30 × 10 -8 ) 参与伤口愈合的基因。
结论和相关性 吡哆醇没有显着预防或延迟 2 级或更高级别 HFS 的发作。血清和红细胞叶酸水平是 HFS 的独立预测因子。
试验注册 临床试验.gov 标识符:NCT00486213



























 京公网安备 11010802027423号
京公网安备 11010802027423号