JAMA Oncology ( IF 22.5 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.1609 Bailiang Li 1 , Yi Cui 1, 2 , Maximilian Diehn 1, 3, 4 , Ruijiang Li 1, 4
|
Importance The prevalence of early-stage non–small cell lung cancer (NSCLC) is expected to increase with recent implementation of annual screening programs. Reliable prognostic biomarkers are needed to identify patients at a high risk for recurrence to guide adjuvant therapy.
Objective To develop a robust, individualized immune signature that can estimate prognosis in patients with early-stage nonsquamous NSCLC.
Design, Setting, and Participants This retrospective study analyzed the gene expression profiles of frozen tumor tissue samples from 19 public NSCLC cohorts, including 18 microarray data sets and 1 RNA-Seq data set for The Cancer Genome Atlas (TCGA) lung adenocarcinoma cohort. Only patients with nonsquamous NSCLC with clinical annotation were included. Samples were from 2414 patients with nonsquamous NSCLC, divided into a meta-training cohort (729 patients), meta-testing cohort (716 patients), and 3 independent validation cohorts (439, 323, and 207 patients). All patients underwent surgery with a negative surgical margin, received no adjuvant or neoadjuvant therapy, and had publicly available gene expression data and survival information. Data were collected from July 22 through September 8, 2016.
Main Outcomes and Measures Overall survival.
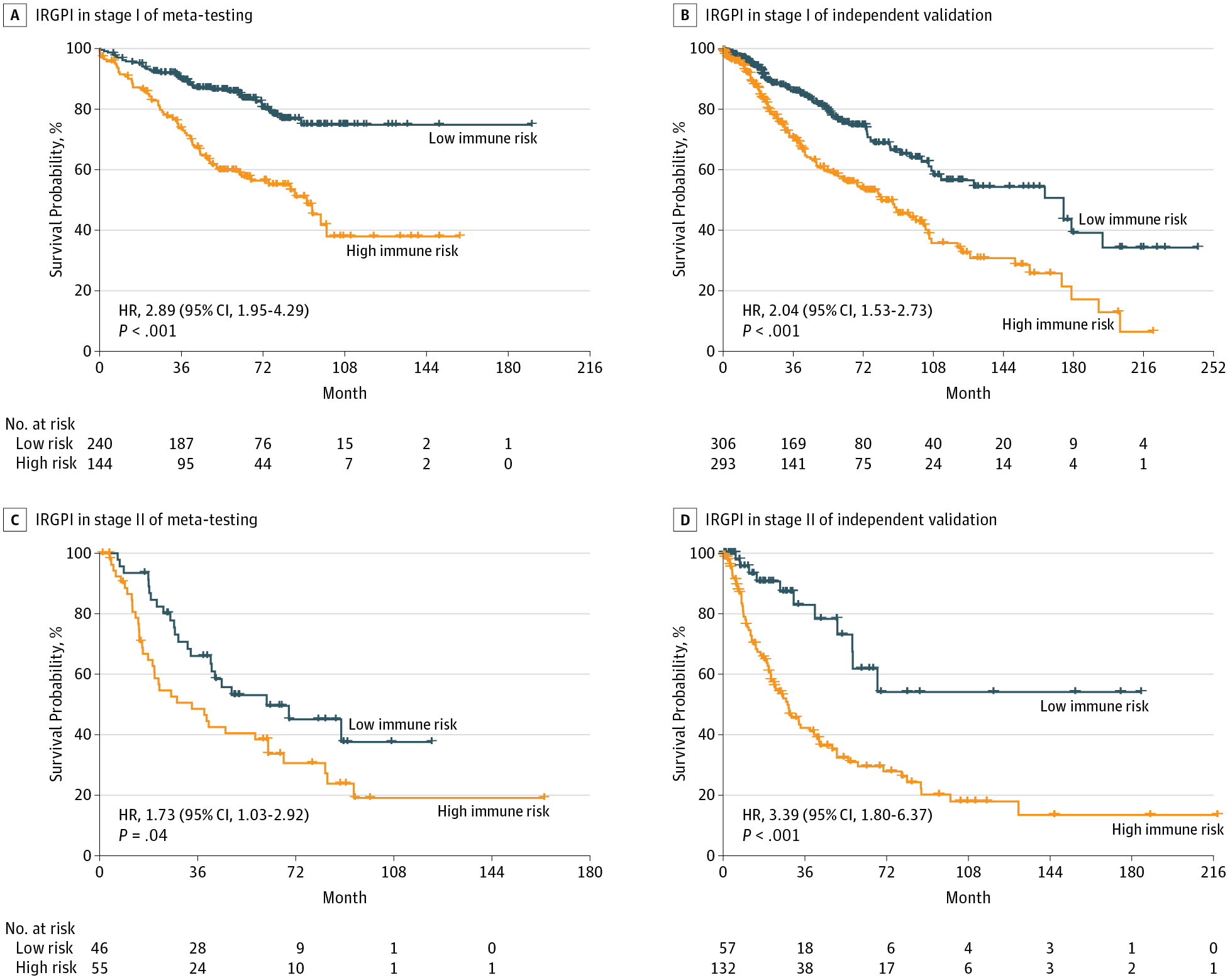
Results Of 2414 patients (1205 men [50%], 1111 women [46%], and 98 of unknown sex [4%]; median age [range], 64 [15-90] years), a prognostic immune signature of 25 gene pairs consisting of 40 unique genes was constructed using the meta-training data set. In the meta-testing and validation cohorts, the immune signature significantly stratified patients into high- vs low-risk groups in terms of overall survival across and within subpopulations with stage I, IA, IB, or II disease and remained as an independent prognostic factor in multivariate analyses (hazard ratio range, 1.72 [95% CI, 1.26-2.33; P < .001] to 2.36 [95% CI, 1.47-3.79; P < .001]) after adjusting for clinical and pathologic factors. Several biological processes, including chemotaxis, were enriched among genes in the immune signature. The percentage of neutrophil infiltration (5.6% vs 1.8%) and necrosis (4.6% vs 1.5%) was significantly higher in the high-risk immune group compared with the low-risk groups in TCGA data set (P < .003). The immune signature achieved a higher accuracy (mean concordance index [C-index], 0.64) than 2 commercialized multigene signatures (mean C-index, 0.53 and 0.61) for estimation of survival in comparable validation cohorts. When integrated with clinical characteristics such as age and stage, the composite clinical and immune signature showed improved prognostic accuracy in all validation data sets relative to molecular signatures alone (mean C-index, 0.70 vs 0.63) and another commercialized clinical-molecular signature (mean C-index, 0.68 vs 0.65).
Conclusions and Relevance The proposed clinical-immune signature is a promising biomarker for estimating overall survival in nonsquamous NSCLC, including early-stage disease. Prospective studies are needed to test the clinical utility of the biomarker in individualized management of nonsquamous NSCLC.
中文翻译:

早期非鳞状非小细胞肺癌个体化免疫预后特征的开发和验证
重要性 随着近期年度筛查计划的实施,早期非小细胞肺癌 (NSCLC) 的患病率预计会增加。需要可靠的预后生物标志物来识别复发风险高的患者,以指导辅助治疗。
目的 开发一种稳健的、个体化的免疫特征,可以估计早期非鳞状 NSCLC 患者的预后。
设计、设置和参与者 这项回顾性研究分析了来自 19 个公共 NSCLC 队列的冷冻肿瘤组织样本的基因表达谱,包括 18 个微阵列数据集和 1 个用于癌症基因组图谱 (TCGA) 肺腺癌队列的 RNA-Seq 数据集。仅包括具有临床注释的非鳞状 NSCLC 患者。样本来自 2414 名非鳞状 NSCLC 患者,分为元训练队列(729 名患者)、元测试队列(716 名患者)和 3 个独立验证队列(439、323 和 207 名患者)。所有患者均接受了手术切缘阴性的手术,未接受辅助或新辅助治疗,并且拥有公开的基因表达数据和生存信息。数据收集时间为 2016 年 7 月 22 日至 9 月 8 日。
主要结果和测量 总体生存率。
结果 2414 名患者(1205 名男性 [50%]、1111 名女性 [46%] 和 98 名未知性别 [4%];中位年龄 [范围],64 [15-90] 岁),预后免疫特征为 25使用元训练数据集构建了由 40 个独特基因组成的基因对。在元测试和验证队列中,就 I、IA、IB 或 II 期疾病亚群之间和内部的总生存率而言,免疫特征显着将患者分为高风险组和低风险组,并且仍然是一个独立的预后因素在多变量分析中(风险比范围,1.72 [95% CI,1.26-2.33;P < .001] 至 2.36 [95% CI,1.47-3.79;P < .001])在调整临床和病理因素后。包括趋化性在内的几个生物学过程在免疫特征的基因中富集。与 TCGA 数据集中的低风险组相比,高危免疫组的中性粒细胞浸润百分比(5.6% vs 1.8%)和坏死(4.6% vs 1.5%)显着更高(P < .003)。在可比较的验证队列中,与 2 个商业化的多基因特征(平均 C 指数,0.53 和 0.61)相比,免疫特征实现了更高的准确度(平均一致性指数 [C 指数],0.64)。当与年龄和分期等临床特征相结合时,复合临床和免疫特征在所有验证数据集中显示出相对于单独的分子特征(平均 C 指数,0.70 对 0.63)和另一个商业化临床分子特征(平均C 指数,0.68 对 0.65)。
结论和相关性 所提出的临床免疫特征是一种有前途的生物标志物,可用于估计包括早期疾病在内的非鳞状 NSCLC 的总生存期。需要前瞻性研究来测试生物标志物在非鳞状 NSCLC 个体化管理中的临床效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号