JAMA Oncology ( IF 22.5 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.1588 Georgina V Long 1, 2 , Jeffrey S Weber 3, 4 , James Larkin 5 , Victoria Atkinson 6 , Jean-Jacques Grob 7 , Dirk Schadendorf 8, 9 , Reinhard Dummer 10 , Caroline Robert 11 , Ivan Márquez-Rodas 12 , Catriona McNeil 13, 14 , Henrik Schmidt 15 , Karen Briscoe 16 , Jean-François Baurain 17 , F Stephen Hodi 18 , Jedd D Wolchok 19
|
Importance Immune checkpoint inhibitors have demonstrated atypical response patterns, which may not be fully captured by conventional response criteria. There is a need to better understand the potential benefit of continued immune checkpoint inhibition beyond progression.
Objective To evaluate the safety and potential benefit of nivolumab (anti–programmed cell death receptor 1) monotherapy beyond Response Evaluation Criteria in Solid Tumors (RECIST) v1.1-defined progression.
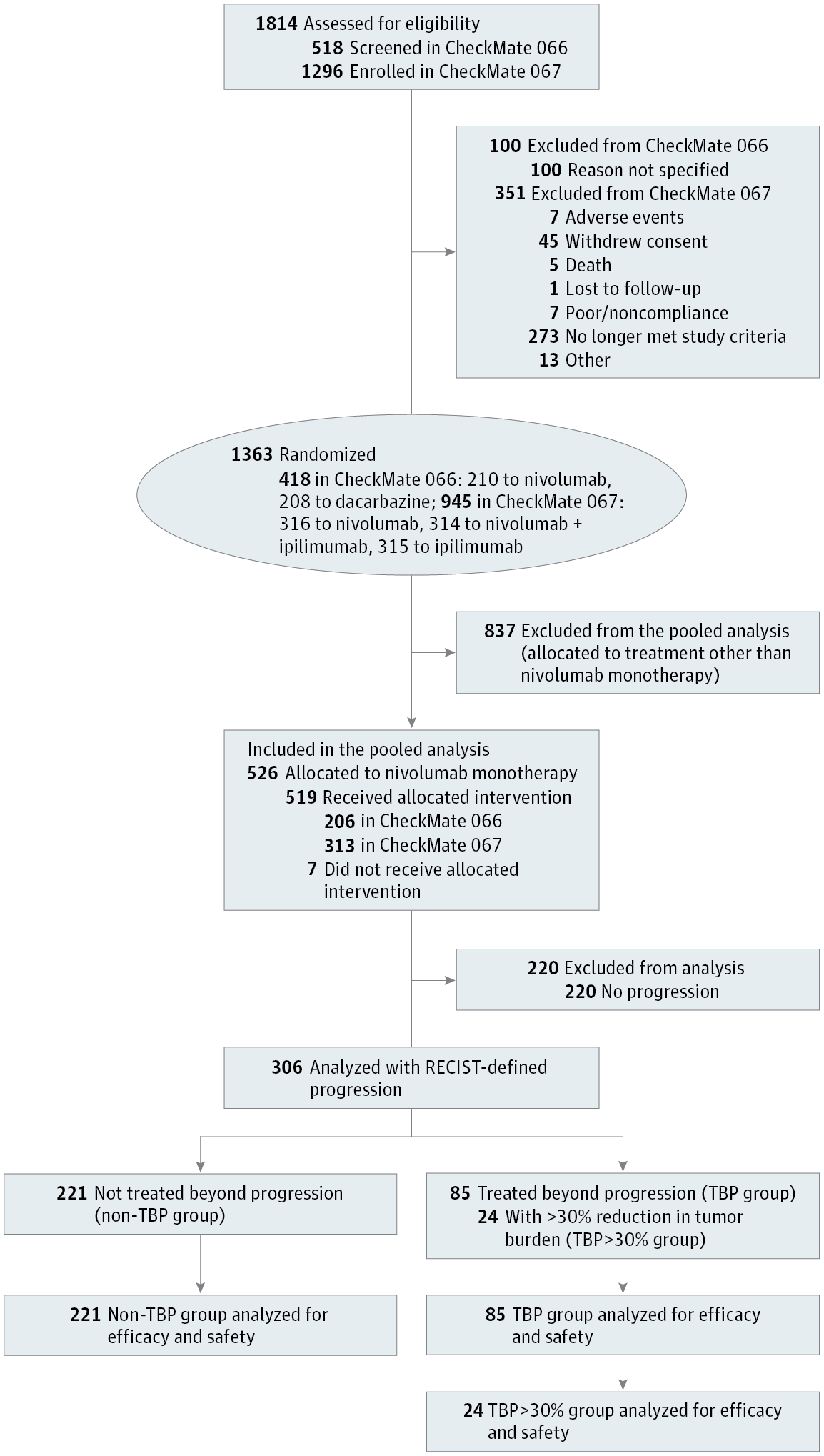
Design, Setting, and Participants Pooled, retrospective analysis of data from phase 3 trials of nivolumab in treatment-naive patients with advanced melanoma (CheckMate 066 or CheckMate 067) conducted at academic and clinical cancer centers. Participants were patients treated beyond first disease progression, defined as those who received their last dose of nivolumab more than 6 weeks after progression (TBP group); and patients not treated beyond progression, who discontinued nivolumab therapy before or at progression (non-TBP group). Data analyses were conducted from November 6, 2015, to January 11, 2017.
Interventions Nivolumab (3 mg/kg every 2 weeks) administered until progression or unacceptable toxic effects. Patients could be treated beyond progression if deriving apparent clinical benefit and tolerating study drug, at the investigator’s discretion.
Main Outcomes and Measures Tumor response and safety in TBP and non-TBP patients.
Results Among 526 randomized patients (39% [n = 203] female; median age, 62 years [range, 18-90 years]), 306 (58%) experienced disease progression, including 85 (28%) TBP patients and 221 (72%) non-TBP patients. Twenty-four (28%) of the TBP patients had a target lesion reduction of greater than 30% after progression compared with baseline (TBP>30% group). At the time of this analysis, 65 (76%) TBP patients and 21 (87%) TBP>30% patients were still alive; 27 (32%) and 11 (46%), respectively, continued to receive treatment. Median (range) time from progression to last dose of treatment was 4.7 (1.4-25.8) months for TBP patients and 7.6 (2.4-19.4) months for TBP>30% patients. Median (range) time from progression to greater than 30% tumor reduction was 1.4 (0.2-7.0) months. Treatment-related select grade 3 to 4 adverse events were similar in the TBP and non-TBP groups (5 [6%] and 9 [4%], respectively).
Conclusions and Relevance A substantial proportion of selected patients treated with frontline nivolumab who were clinically stable and judged to be eligible for treatment beyond RECIST v1.1–defined progression by the treating investigators derived apparent clinical benefit without compromising safety. Further analysis will help define the potential benefit of continued nivolumab treatment beyond progression.
Trial Registration clinicaltrials.gov Identifiers: NCT01721772 (CheckMate 066) and NCT01844505 (CheckMate 067)
中文翻译:

Nivolumab 治疗晚期黑色素瘤患者超越进展分析 2 项 3 期临床试验
重要性 免疫检查点抑制剂已表现出非典型反应模式,传统反应标准可能无法完全捕捉到这种模式。有必要更好地了解持续免疫检查点抑制在进展之外的潜在益处。
目的 评估 nivolumab(抗程序性细胞死亡受体 1)单药治疗超出实体瘤反应评估标准 (RECIST) v1.1 定义的进展的安全性和潜在益处。
设计、设置和参与者 对在学术和临床癌症中心进行的 nivolumab 治疗初治晚期黑色素瘤患者(CheckMate 066 或 CheckMate 067)的 3 期试验数据进行汇总、回顾性分析。参与者是在首次疾病进展后接受治疗的患者,定义为在疾病进展后 6 周以上接受最后一剂纳武单抗的患者(TBP 组);进展前或进展时停止纳武利尤单抗治疗的患者(非 TBP 组)。数据分析于 2015 年 11 月 6 日至 2017 年 1 月 11 日进行。
干预 Nivolumab(每 2 周 3 mg/kg)给药直至进展或出现不可接受的毒性作用。如果获得明显的临床益处并耐受研究药物,则可以根据研究人员的判断对患者进行治疗。
主要结果和措施 TBP 和非 TBP 患者的肿瘤反应和安全性。
结果 在 526 名随机患者(39% [n = 203] 女性;中位年龄,62 岁 [范围,18-90 岁])中,306 名(58%)患者出现疾病进展,包括 85 名(28%)TBP 患者和 221 名(72 %) 非 TBP 患者。与基线相比(TBP>30% 组),24 名 (28%) 的 TBP 患者在进展后的目标病变减少超过 30%。在本次分析时,65 名 (76%) 的 TBP 患者和 21 名 (87%) 的 TBP>30% 的患者仍然存活;分别有 27 人 (32%) 和 11 人 (46%) 继续接受治疗。TBP 患者从进展到最后一次治疗的中位(范围)时间为 4.7(1.4-25.8)个月,TBP>30% 患者为 7.6(2.4-19.4)个月。从进展到肿瘤缩小超过 30% 的中位(范围)时间为 1.4(0.2-7.0)个月。
结论和相关性 接受一线 nivolumab 治疗的选定患者中有相当一部分临床稳定并被治疗研究人员判断为有资格接受 RECIST v1.1 定义的进展后的治疗,这些患者在不影响安全性的情况下获得了明显的临床益处。进一步的分析将有助于确定继续纳武利尤单抗治疗超越进展的潜在益处。
试验注册 clinicaltrials.gov 标识符:NCT01721772 (CheckMate 066) 和NCT01844505 (CheckMate 067)











































 京公网安备 11010802027423号
京公网安备 11010802027423号