JAMA Oncology ( IF 22.5 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamaoncol.2017.1819 Gideon M Blumenthal 1 , Marc R Theoret 1 , Richard Pazdur 1, 2
|
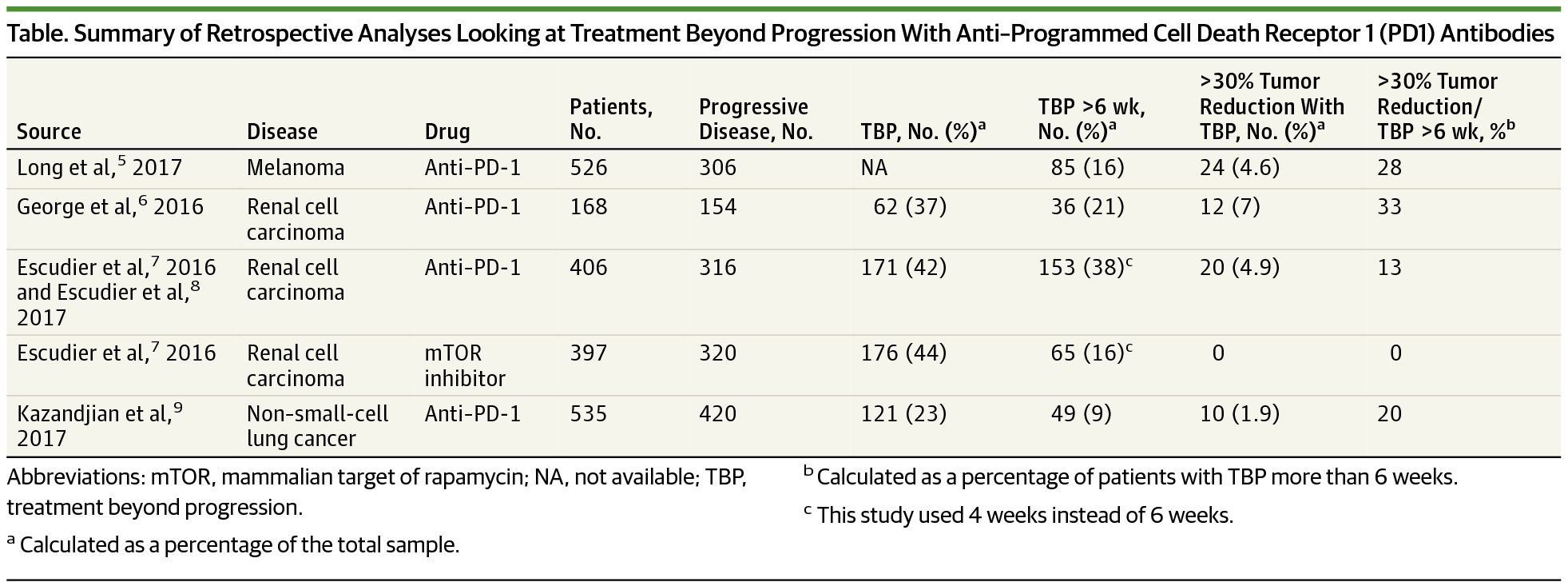
The development and subsequent approval of antibodies against the immune regulators programmed cell death receptor 1 (PD-1) and its ligand PD-L1 is changing treatment paradigms in a variety of cancers, with 5 of these antibodies approved to date.1 Whereas immune checkpoint inhibitor development is among the most successful and heavily investigated areas in oncology drug development, several uncertainties remain regarding how best to use these therapies in clinical practice.2 Important questions include how best to predict, identify, and optimally manage immune-related adverse events, the utility of complementary or companion diagnostic tests to predict which patients will and will not benefit, optimal dosing and scheduling, and the optimal duration of treatment.
中文翻译:

免疫检查点抑制剂超越进展的治疗——已知的未知数
针对免疫调节剂程序性细胞死亡受体 1 (PD-1) 及其配体 PD-L1 的抗体的开发和随后的批准正在改变各种癌症的治疗模式,其中 5 种抗体迄今已获批准。1虽然免疫检查点抑制剂的开发是肿瘤药物开发中最成功和研究最深入的领域之一,但在临床实践中如何最好地使用这些疗法仍存在一些不确定性。2重要问题包括如何最好地预测、识别和优化管理与免疫相关的不良事件,补充或伴随诊断测试预测哪些患者将受益和不会受益的效用,最佳剂量和时间安排,以及最佳治疗持续时间。










































 京公网安备 11010802027423号
京公网安备 11010802027423号