当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Correlating in Vitro Solubilization and Supersaturation Profiles with in Vivo Exposure for Lipid Based Formulations of the CETP Inhibitor CP-532,623
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00660 Claire L. McEvoy , Natalie L. Trevaskis , Orlagh M. Feeney , Glenn A. Edwards 1 , Michael E. Perlman 2 , Catherine M. Ambler 2 , Christopher J. H. Porter
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00660 Claire L. McEvoy , Natalie L. Trevaskis , Orlagh M. Feeney , Glenn A. Edwards 1 , Michael E. Perlman 2 , Catherine M. Ambler 2 , Christopher J. H. Porter
Affiliation
|
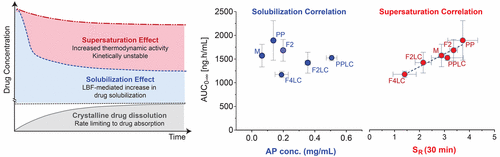
Lipid based formulations (LBFs) are a promising formulation strategy for many poorly water-soluble drugs and have been shown previously to enhance the oral exposure of CP-532,623, an oral cholesteryl ester transfer protein inhibitor. In the current study, an in vitro lipid digestion model was used to probe the relationship between drug solubilization and supersaturation on in vitro dispersion and digestion of LBF containing long chain (LC) lipids and drug absorption in vivo. After in vitro digestion of LBF based on LC lipids, the proportion of CP-532,623 maintained in the solubilized state in the aqueous phase of the digest was highest in formulations containing Kolliphor RH 40, and in most cases outperformed equivalent formulations based on MC lipids. Subsequent administration of the LC-LBFs to beagle dogs resulted in reasonable correlation between concentrations of CP-532,623 measured in the aqueous phase of the in vitro digest after 30 min digestion and in vivo exposure (AUC); however, the LC-LBFs required greater in vitro drug solubilization to elicit similar in vivo exposure when compared to previous studies with MC-LBF. Although post digestion solubilization was enhanced in LC-LBF compared to MC-LBF, equilibrium solubility studies of CP-532,623 in the aqueous phase isolated from blank lipid digestion experiments revealed that equilibrium solubility was also higher, and therefore supersaturation lower. A revised correlation based on supersaturation in the digest aqueous phase and drug absorption was therefore generated. A single, linear correlation was evident for both LC- and MC-LBF containing Kolliphor RH 40, but this did not extend to formulations based on other surfactants. The data suggest that solubilization and supersaturation are significant drivers of drug absorption in vivo, and that across formulations with similar formulation composition good correlation is evident between in vitro and in vivo measures. However, across dissimilar formulations, solubilization and supersaturation alone are not sufficient to explain drug exposure and other factors also likely play a role.
中文翻译:

CETP抑制剂CP-532,623基于脂质的制剂的体外增溶和过饱和曲线与体内暴露相关
基于脂质的制剂(LBF)是许多水溶性差的药物的有前途的制剂策略,并且先前已证明可增强口服胆固醇酯转移蛋白抑制剂CP-532,623的口服暴露量。在当前的研究中,使用体外脂质消化模型来探讨药物的溶解和过饱和之间的关系,所述体外溶解和过饱和对包含长链(LC)脂质的LBF脂质的体外分散和消化以及体内药物的吸收。体外消化基于LC脂质的LBF后,在含有Kolliphor RH 40的配方中,在消化液水相中保持增溶状态的CP-532,623的比例最高,在大多数情况下,其性能优于基于MC脂质的等效配方。随后向比格犬施用LC-LBF,导致30分钟消化后体外消化液水相中测得的CP-532,623浓度与体内暴露(AUC)之间存在合理的相关性;然而,与以前的MC-LBF研究相比,LC-LBF需要更大的体外药物增溶作用以引起相似的体内暴露。尽管与MC-LBF相比,LC-LBF中的消化后溶解度得到了增强,但是从空白脂质消化实验中分离出的水相中CP-532,623的平衡溶解度研究表明,平衡溶解度也较高,因此过饱和度较低。因此,产生了基于消化水相中过饱和度和药物吸收的修正相关性。一个,含有Kolliphor RH 40的LC-和MC-LBF均具有线性相关性,但这并没有扩展到基于其他表面活性剂的配方中。数据表明增溶和过饱和是体内药物吸收的重要驱动力,并且在具有相似制剂组成的整个制剂中,在体外和体内测量之间有很好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。并且在具有相似配方组成的所有配方之间,在体外和体内测量之间具有良好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。并且在具有相似配方组成的所有配方之间,在体外和体内测量之间具有良好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。
更新日期:2017-11-09
中文翻译:

CETP抑制剂CP-532,623基于脂质的制剂的体外增溶和过饱和曲线与体内暴露相关
基于脂质的制剂(LBF)是许多水溶性差的药物的有前途的制剂策略,并且先前已证明可增强口服胆固醇酯转移蛋白抑制剂CP-532,623的口服暴露量。在当前的研究中,使用体外脂质消化模型来探讨药物的溶解和过饱和之间的关系,所述体外溶解和过饱和对包含长链(LC)脂质的LBF脂质的体外分散和消化以及体内药物的吸收。体外消化基于LC脂质的LBF后,在含有Kolliphor RH 40的配方中,在消化液水相中保持增溶状态的CP-532,623的比例最高,在大多数情况下,其性能优于基于MC脂质的等效配方。随后向比格犬施用LC-LBF,导致30分钟消化后体外消化液水相中测得的CP-532,623浓度与体内暴露(AUC)之间存在合理的相关性;然而,与以前的MC-LBF研究相比,LC-LBF需要更大的体外药物增溶作用以引起相似的体内暴露。尽管与MC-LBF相比,LC-LBF中的消化后溶解度得到了增强,但是从空白脂质消化实验中分离出的水相中CP-532,623的平衡溶解度研究表明,平衡溶解度也较高,因此过饱和度较低。因此,产生了基于消化水相中过饱和度和药物吸收的修正相关性。一个,含有Kolliphor RH 40的LC-和MC-LBF均具有线性相关性,但这并没有扩展到基于其他表面活性剂的配方中。数据表明增溶和过饱和是体内药物吸收的重要驱动力,并且在具有相似制剂组成的整个制剂中,在体外和体内测量之间有很好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。并且在具有相似配方组成的所有配方之间,在体外和体内测量之间具有良好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。并且在具有相似配方组成的所有配方之间,在体外和体内测量之间具有良好的相关性。但是,在不同的配方中,仅增溶和过饱和不足以解释药物暴露,其他因素也可能起作用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号