Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicentre, randomised, phase 3 trial.
The Lancet ( IF 168.9 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1470-2045(17)30691-5 Heather A Wakelee 1 , Suzanne E Dahlberg 2 , Steven M Keller 3 , William J Tester 4 , David R Gandara 5 , Stephen L Graziano 6 , Alex A Adjei 7 , Natasha B Leighl 8 , Seena C Aisner 9 , Jan M Rothman 10 , Jyoti D Patel 11 , Mark D Sborov 12 , Sean R McDermott 13 , Roman Perez-Soler 14 , Anne M Traynor 15 , Charles Butts 16 , Tracey Evans 17 , Atif Shafqat 18 , Andrew E Chapman 19 , Samer S Kasbari 20 , Leora Horn 21 , Suresh S Ramalingam 22 , Joan H Schiller 23 ,
The Lancet ( IF 168.9 ) Pub Date : 2017-12-01 , DOI: 10.1016/s1470-2045(17)30691-5 Heather A Wakelee 1 , Suzanne E Dahlberg 2 , Steven M Keller 3 , William J Tester 4 , David R Gandara 5 , Stephen L Graziano 6 , Alex A Adjei 7 , Natasha B Leighl 8 , Seena C Aisner 9 , Jan M Rothman 10 , Jyoti D Patel 11 , Mark D Sborov 12 , Sean R McDermott 13 , Roman Perez-Soler 14 , Anne M Traynor 15 , Charles Butts 16 , Tracey Evans 17 , Atif Shafqat 18 , Andrew E Chapman 19 , Samer S Kasbari 20 , Leora Horn 21 , Suresh S Ramalingam 22 , Joan H Schiller 23 ,
Affiliation
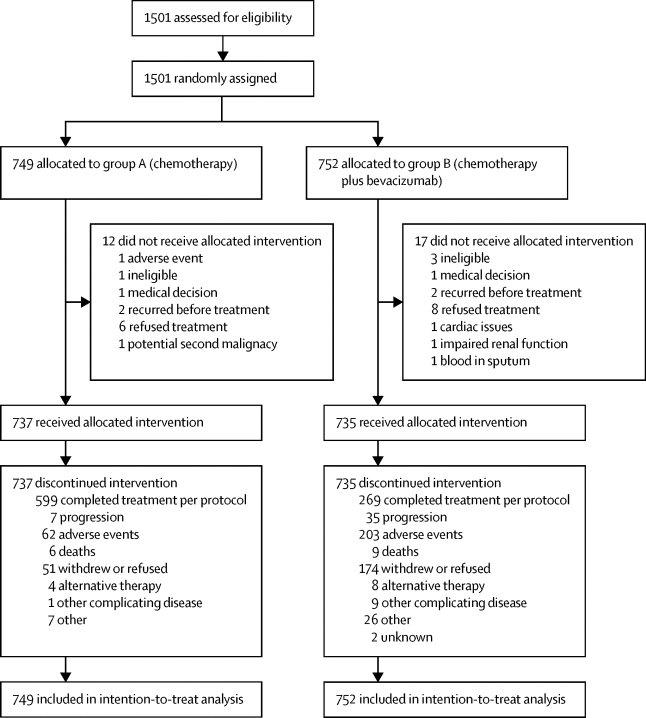
|
Adjuvant chemotherapy for resected early-stage non-small-cell lung cancer (NSCLC) provides a modest survival benefit. Bevacizumab, a monoclonal antibody directed against VEGF, improves outcomes when added to platinum-based chemotherapy in advanced-stage non-squamous NSCLC. We aimed to evaluate the addition of bevacizumab to adjuvant chemotherapy in early-stage resected NSCLC.
中文翻译:

已切除的非小细胞肺癌患者联合或不联合贝伐珠单抗的辅助化疗 (E1505):一项开放标签、多中心、随机、3 期试验。
对切除的早期非小细胞肺癌(NSCLC)进行辅助化疗可提供适度的生存获益。贝伐单抗是一种针对 VEGF 的单克隆抗体,添加到晚期非鳞状 NSCLC 的铂类化疗中可改善预后。我们的目的是评估在早期切除的非小细胞肺癌的辅助化疗中添加贝伐珠单抗。
更新日期:2017-11-30
中文翻译:

已切除的非小细胞肺癌患者联合或不联合贝伐珠单抗的辅助化疗 (E1505):一项开放标签、多中心、随机、3 期试验。
对切除的早期非小细胞肺癌(NSCLC)进行辅助化疗可提供适度的生存获益。贝伐单抗是一种针对 VEGF 的单克隆抗体,添加到晚期非鳞状 NSCLC 的铂类化疗中可改善预后。我们的目的是评估在早期切除的非小细胞肺癌的辅助化疗中添加贝伐珠单抗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号