当前位置:
X-MOL 学术
›
RSC Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic antifungal effect of cyclized chalcone derivatives and fluconazole against Candida albicans
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7md00440k Aijaz Ahmad 1, 2, 3, 4, 5 , Mohmmad Younus Wani 6, 7, 8, 9, 10 , Mrudula Patel 3, 4, 5, 11, 12 , Abilio J. F. N. Sobral 10, 13, 14, 15, 16 , Adriano G. Duse 1, 2, 3, 4, 5 , Faisal Mohammed Aqlan 17, 18, 19, 20, 21 , Abdullah Saad Al-Bogami 17, 18, 19, 20, 21
RSC Medicinal Chemistry ( IF 4.1 ) Pub Date : 2017-10-17 00:00:00 , DOI: 10.1039/c7md00440k Aijaz Ahmad 1, 2, 3, 4, 5 , Mohmmad Younus Wani 6, 7, 8, 9, 10 , Mrudula Patel 3, 4, 5, 11, 12 , Abilio J. F. N. Sobral 10, 13, 14, 15, 16 , Adriano G. Duse 1, 2, 3, 4, 5 , Faisal Mohammed Aqlan 17, 18, 19, 20, 21 , Abdullah Saad Al-Bogami 17, 18, 19, 20, 21
Affiliation
|
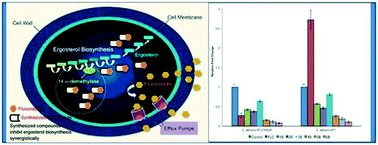
The occurrence of invasive fungal diseases, particularly in immunocompromised patients, is life-threatening and increases the economic burden. The rising problem of multi-drug resistance is becoming a major concern for clinicians. In addition, a repertoire of antifungal agents is far less in number than antibacterial drugs. To combat these problems, combination therapy has gained a lot of interest. We previously reported the synergistic interaction of some mono- and bis-dihydropyrimidinone and thione derivatives with fluconazole and amphotericin B for combination antifungal therapy. In this study we used the same approach and synthesized different azole and non-azole derivatives of mono-(M) and bis-(B) chalcones and evaluated their antifungal activity profile alone and in combination with the most commonly used antifungal drug – fluconazole (FLC) – against seven FLC susceptible and three FLC resistant clinically isolated Candida albicans strains. Based on the minimum inhibitory concentration results, the bis-derivatives showed lower MIC values compared to their mono-analogues. Both fractional inhibitory concentration index and isobologram results revealed mostly synergistic, additive or indifferent interactions between the tested compounds and FLC against different Candida isolates. None of the tested compounds showed any effect on energy dependent R6G efflux, revealing that they do not reverse the mechanism of drug efflux. However, surprisingly, these compounds profoundly decreased ergosterol biosynthesis and showed down regulation of ERG11 gene expression, which is the possible mechanism of reversal of azole drug resistance by these compounds. These results provide a platform for further research to develop pyrimidinone/thione ring containing compounds as promising new antifungal agents, which could be used in antifungal combination therapy.
中文翻译:

环查尔酮衍生物和氟康唑对白色念珠菌的协同抗真菌作用
侵袭性真菌疾病的发生,特别是在免疫功能低下的患者中,危及生命,并增加了经济负担。日益增长的多药耐药性问题已成为临床医生关注的主要问题。此外,抗真菌剂的种类远远少于抗菌药物。为了解决这些问题,组合疗法引起了很多兴趣。我们先前报道了一些单-和双-二氢嘧啶酮和硫酮衍生物与氟康唑和两性霉素B的协同相互作用,以进行联合抗真菌治疗。在这项研究中,我们使用相同的方法并合成了单-(M)和双-(B)查耳酮并单独评估其抗真菌活性谱,并与最常用的抗真菌药fluconazole(FLC)结合使用,针对7种易感FLC和3种临床耐药的白色念珠菌菌株进行抗真菌活性评估。根据最小抑菌浓度结果,双衍生物比单模拟物显示出较低的MIC值。抑制分数浓度指数和等效线图结果均显示,受试化合物与FLC之间针对不同念珠菌的协同,加性或无关性相互作用隔离株。所测试的化合物均未显示出对能量依赖性R6G外排的任何影响,表明它们并未逆转药物外排的机制。然而,令人惊讶的是,这些化合物极大地降低了麦角固醇的生物合成并显示出ERG11基因表达的下调,这是这些化合物逆转对唑耐药性的可能机制。这些结果为进一步开发含嘧啶酮/硫酮环的化合物作为有希望的新型抗真菌剂提供了平台,可将其用于抗真菌联合治疗。
更新日期:2017-11-08
中文翻译:

环查尔酮衍生物和氟康唑对白色念珠菌的协同抗真菌作用
侵袭性真菌疾病的发生,特别是在免疫功能低下的患者中,危及生命,并增加了经济负担。日益增长的多药耐药性问题已成为临床医生关注的主要问题。此外,抗真菌剂的种类远远少于抗菌药物。为了解决这些问题,组合疗法引起了很多兴趣。我们先前报道了一些单-和双-二氢嘧啶酮和硫酮衍生物与氟康唑和两性霉素B的协同相互作用,以进行联合抗真菌治疗。在这项研究中,我们使用相同的方法并合成了单-(M)和双-(B)查耳酮并单独评估其抗真菌活性谱,并与最常用的抗真菌药fluconazole(FLC)结合使用,针对7种易感FLC和3种临床耐药的白色念珠菌菌株进行抗真菌活性评估。根据最小抑菌浓度结果,双衍生物比单模拟物显示出较低的MIC值。抑制分数浓度指数和等效线图结果均显示,受试化合物与FLC之间针对不同念珠菌的协同,加性或无关性相互作用隔离株。所测试的化合物均未显示出对能量依赖性R6G外排的任何影响,表明它们并未逆转药物外排的机制。然而,令人惊讶的是,这些化合物极大地降低了麦角固醇的生物合成并显示出ERG11基因表达的下调,这是这些化合物逆转对唑耐药性的可能机制。这些结果为进一步开发含嘧啶酮/硫酮环的化合物作为有希望的新型抗真菌剂提供了平台,可将其用于抗真菌联合治疗。











































 京公网安备 11010802027423号
京公网安备 11010802027423号