当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The diaza-Nazarov cyclization involving a 2,3-diaza-pentadienyl cation for the synthesis of polysubstituted pyrazoles
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7ob01949a Balakrishna Aegurla 1, 2, 3 , Rama Krishna Peddinti 1, 2, 3
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1039/c7ob01949a Balakrishna Aegurla 1, 2, 3 , Rama Krishna Peddinti 1, 2, 3
Affiliation
|
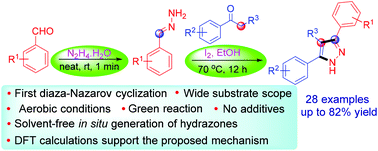
An unprecedented iodine-mediated diaza-Nazarov (DAN) type cyclization for the construction of substituted pyrazoles from easily available starting materials via an enamine–iminium ion intermediate is described. The oxidative cyclization worked under green conditions with remarkable regioselectivity. This one-pot, efficient and operationally simple three-component intramolecular regioselective DAN cyclization displayed a wide range of substrate scope. The dichotomy of reaction pathways has been explored with density functional theory in the gas phase and solution phase. Of the possible 1,5-, 1,6-, and 1,7-electrocyclizations, the DAN cyclization, i.e., the 1,5-pathway offers the lowest activation energy barrier supporting our experimental observations.
中文翻译:

涉及2,3-二氮杂戊二烯基阳离子的diaza-Nazarov环化反应,用于合成多取代的吡唑
描述了前所未有的碘介导的diaza-Nazarov(DAN)型环化反应,该反应可通过易用的起始原料通过烯胺-亚胺离子中间体构建取代的吡唑。氧化环化反应在绿色条件下具有显着的区域选择性。这种一锅,高效且操作简单的三组分分子内区域选择性DAN环化反应显示了广泛的底物范围。已经用密度泛函理论在气相和溶液相中探索了反应途径的二分法。在可能的1,5-,1,6-和1,7-电环化中,DAN环化(即1,5-途径)提供了最低的活化能垒,支持了我们的实验观察。
更新日期:2017-11-08
中文翻译:

涉及2,3-二氮杂戊二烯基阳离子的diaza-Nazarov环化反应,用于合成多取代的吡唑
描述了前所未有的碘介导的diaza-Nazarov(DAN)型环化反应,该反应可通过易用的起始原料通过烯胺-亚胺离子中间体构建取代的吡唑。氧化环化反应在绿色条件下具有显着的区域选择性。这种一锅,高效且操作简单的三组分分子内区域选择性DAN环化反应显示了广泛的底物范围。已经用密度泛函理论在气相和溶液相中探索了反应途径的二分法。在可能的1,5-,1,6-和1,7-电环化中,DAN环化(即1,5-途径)提供了最低的活化能垒,支持了我们的实验观察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号