当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ether formation through reductive coupling of ketones or aldehydes catalyzed by a mesoionic carbene iridium complex
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1039/c7cy01832k A. Petronilho 1, 2, 3, 4 , A. Vivancos 5, 6, 7, 8 , M. Albrecht 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-10-20 00:00:00 , DOI: 10.1039/c7cy01832k A. Petronilho 1, 2, 3, 4 , A. Vivancos 5, 6, 7, 8 , M. Albrecht 1, 2, 3, 4, 5
Affiliation
|
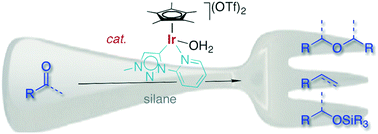
An iridium(III) Cp* complex containing a triazolylidene–pyridyl C,N-bidentate-coordinating ligand is a very powerful catalyst for the transformation of ketones and aldehydes into symmetrical ethers. This highly efficient reductive coupling proceeds immediately at room temperature and at a low catalyst loading (0.1 mol%) when Ph2SiH2 is used as an additive. Aromatic carbonyl substrates react faster than aliphatic ketones or aldehydes, and the substrate scope suggests some functional group tolerance. Likewise, the condensation of alcohols to symmetrical ethers is catalyzed by this triazolylidene iridium complex, though ether formation is an order of magnitude slower than when starting from the analogous ketone or aldehyde as a substrate, suggesting that alcohols are not potential intermediates in the reductive coupling process. Prolonged reactions or modification of the silane additive lead to ether cleavage and dehydration, thus affording the corresponding olefin. Mechanistic insights and in particular the different reactivities of alcohols and ketones have been exploited to develop a synthetic methodology for the iridium-catalyzed formation of unsymmetrical methyl ethers (R–OMe) in good yields.
中文翻译:

通过介电卡宾铱配合物催化的酮或醛的还原偶联形成醚
含有三唑基亚甲基-吡啶基C,N-双齿配位的配体的铱(III)Cp *络合物对于将酮和醛转化为对称醚是非常强大的催化剂。当Ph 2 SiH 2时,这种高效的还原偶联反应可在室温和低催化剂负载量(0.1摩尔%)下立即进行。用作添加剂。芳族羰基底物的反应比脂肪族酮或醛快,并且底物范围表明某些官能团耐受性。同样,醇的缩合反应是由三唑基铱配合物催化的,尽管醚的形成比以类似的酮或醛为底物起始时要慢一个数量级,这表明醇不是还原偶联的潜在中间体。过程。硅烷添加剂的长时间反应或改性会导致醚裂解和脱水,从而得到相应的烯烃。
更新日期:2017-11-08
中文翻译:

通过介电卡宾铱配合物催化的酮或醛的还原偶联形成醚
含有三唑基亚甲基-吡啶基C,N-双齿配位的配体的铱(III)Cp *络合物对于将酮和醛转化为对称醚是非常强大的催化剂。当Ph 2 SiH 2时,这种高效的还原偶联反应可在室温和低催化剂负载量(0.1摩尔%)下立即进行。用作添加剂。芳族羰基底物的反应比脂肪族酮或醛快,并且底物范围表明某些官能团耐受性。同样,醇的缩合反应是由三唑基铱配合物催化的,尽管醚的形成比以类似的酮或醛为底物起始时要慢一个数量级,这表明醇不是还原偶联的潜在中间体。过程。硅烷添加剂的长时间反应或改性会导致醚裂解和脱水,从而得到相应的烯烃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号