当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2O2-responsive and plaque-penetrating nanoplatform for mTOR gene silencing with robust anti-atherosclerosis efficacy
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7sc03582a Wen Gao 1, 2, 3, 4, 5 , Yujie Zhao 1, 2, 3, 4, 5 , Xiang Li 1, 2, 3, 4, 5 , Yuhui Sun 1, 2, 3, 4, 5 , Michelle Cai 6, 7, 8, 9 , Wenhua Cao 1, 2, 3, 4, 5 , Zhenhua Liu 1, 2, 3, 4, 5 , Lili Tong 1, 2, 3, 4, 5 , Guanwei Cui 1, 2, 3, 4, 5 , Bo Tang 1, 2, 3, 4, 5
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-27 00:00:00 , DOI: 10.1039/c7sc03582a Wen Gao 1, 2, 3, 4, 5 , Yujie Zhao 1, 2, 3, 4, 5 , Xiang Li 1, 2, 3, 4, 5 , Yuhui Sun 1, 2, 3, 4, 5 , Michelle Cai 6, 7, 8, 9 , Wenhua Cao 1, 2, 3, 4, 5 , Zhenhua Liu 1, 2, 3, 4, 5 , Lili Tong 1, 2, 3, 4, 5 , Guanwei Cui 1, 2, 3, 4, 5 , Bo Tang 1, 2, 3, 4, 5
Affiliation
|
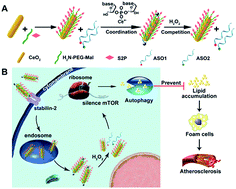
The mammalian target of rapamycin (mTOR) that controls autophagy and lipid metabolism is pivotal for atherosclerosis initiation and progression. Although blocking the mTOR function with rapamycin and its analogs may stimulate autophagy and consequently attenuate lipid storage and atherosclerotic lesions, only limited success has been achieved in clinical applications due to the unsatisfactory efficacy and safety profiles. In this study, we engineered a cerium oxide nanowire (CeO2 NW)-based RNA interference (RNAi) oligonucleotide delivery nanoplatform for the effective silencing of mTOR and treatment of atherosclerosis. This nanoplatform is composed of the following three key components: (i) a stabilin-2-specific peptide ligand (S2P) to improve plaque targeting and penetration; (ii) polyethylene glycosylation (PEGylation) to extend in vivo circulation time; and (iii) a high aspect ratio CeO2 core to facilitate endosome escape and ensure “on-demand” release of the RNAi payloads through competitive coordination of cytosolic hydrogen peroxide (H2O2). Systemic administration of the nanoplatforms efficiently targeted stabilin-2-expressing plaque and suppressed mTOR expression, which significantly rescued the impaired autophagy and inhibited the atherosclerotic lesion progression in apolipoprotein E-deficient (ApoE−/−) mice fed with a high-fat diet. These results demonstrated that this H2O2-responsive and plaque-penetrating nanoplatform can be a potent and safe tool for gene therapy of atherosclerosis.
中文翻译:

H 2 O 2响应和噬斑穿透性纳米平台,用于mTOR基因沉默,具有强大的抗动脉粥样硬化功效
控制自噬和脂质代谢的雷帕霉素(mTOR)哺乳动物靶点对于动脉粥样硬化的发生和发展至关重要。尽管用雷帕霉素及其类似物阻断mTOR功能可能会刺激自噬并因此减弱脂质存储和动脉粥样硬化病变,但由于疗效和安全性不理想,在临床应用中仅取得了有限的成功。在这项研究中,我们设计了氧化铈纳米线(CeO 2基于NW)的RNA干扰(RNAi)寡核苷酸递送纳米平台,可有效沉默mTOR和治疗动脉粥样硬化。该纳米平台由以下三个关键成分组成:(i)稳定蛋白2特异性肽配体(S2P),用于改善噬斑的靶向性和渗透性;(ii)聚乙烯糖基化(PEGylation)以延长体内循环时间;(iii)高纵横比的CeO 2核心,通过竞争性调节胞质过氧化氢(H 2 O 2)促进内体逃逸并确保“按需”释放RNAi负载)。纳米平台的系统性给药有效地靶向表达stabilin-2的噬菌斑并抑制了mTOR表达,从而显着挽救了以高脂饮食喂养的载脂蛋白E缺乏症(ApoE - / -)小鼠的自噬受损并抑制了动脉粥样硬化病变的进展。这些结果表明,这种H 2 O 2响应性和斑块穿透性纳米平台可以成为用于动脉粥样硬化基因治疗的有效且安全的工具。
更新日期:2017-11-08
中文翻译:

H 2 O 2响应和噬斑穿透性纳米平台,用于mTOR基因沉默,具有强大的抗动脉粥样硬化功效
控制自噬和脂质代谢的雷帕霉素(mTOR)哺乳动物靶点对于动脉粥样硬化的发生和发展至关重要。尽管用雷帕霉素及其类似物阻断mTOR功能可能会刺激自噬并因此减弱脂质存储和动脉粥样硬化病变,但由于疗效和安全性不理想,在临床应用中仅取得了有限的成功。在这项研究中,我们设计了氧化铈纳米线(CeO 2基于NW)的RNA干扰(RNAi)寡核苷酸递送纳米平台,可有效沉默mTOR和治疗动脉粥样硬化。该纳米平台由以下三个关键成分组成:(i)稳定蛋白2特异性肽配体(S2P),用于改善噬斑的靶向性和渗透性;(ii)聚乙烯糖基化(PEGylation)以延长体内循环时间;(iii)高纵横比的CeO 2核心,通过竞争性调节胞质过氧化氢(H 2 O 2)促进内体逃逸并确保“按需”释放RNAi负载)。纳米平台的系统性给药有效地靶向表达stabilin-2的噬菌斑并抑制了mTOR表达,从而显着挽救了以高脂饮食喂养的载脂蛋白E缺乏症(ApoE - / -)小鼠的自噬受损并抑制了动脉粥样硬化病变的进展。这些结果表明,这种H 2 O 2响应性和斑块穿透性纳米平台可以成为用于动脉粥样硬化基因治疗的有效且安全的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号