当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solid Versus Solution Spin Crossover and the Importance of the Fe–N≡C(X) Angle
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01338 Santiago Rodríguez-Jiménez 1 , Sally Brooker 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01338 Santiago Rodríguez-Jiménez 1 , Sally Brooker 1
Affiliation
|
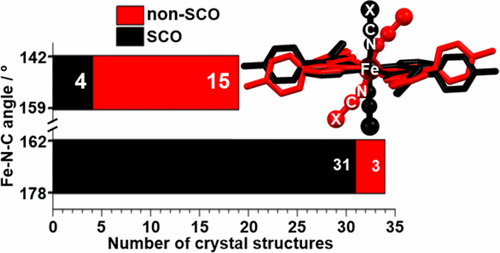
A new family of mononuclear [FeII(Rdpt)2(NCE)2] complexes (E = S, Se, or BH3) is formed by 1:2 reaction of [FeII(pyridine)4(NCE)2] with the monotopic pyridyl triazole ligand 4-(4-methylphenyl)-3-(2-pyridinyl)-5-phenyl-4H-1,2,4-triazole (tolpyph). The three complexes are obtained as six different solvatomorphs: [FeII(tolpyph)2(NCS)2]·H2O (1·H2O), 1·1.5CH3OH·0.5H2O, [FeII(tolpyph)2(NCSe)2] (2), 2·1.5H2O, [FeII(tolpyph)2(NCBH3)2] (3), and 3·H2O. Single-crystal X-ray diffraction reveals that 1·1.5CH3OH·0.5H2O and 2 are high-spin (HS) at 100 K, while 3 is low-spin (LS) at 100 K and HS at 373 K. Compound 3 is the first structurally characterized example of an [FeII(Rdpt)2(NCE)2]-type complex with NCBH3 co-ligand: the crystal packing is dominated by aromatic stacking interactions. Solid-state magnetic measurements show that 1·H2O and 2·1.5H2O remain HS down to 50 K, whereas 3·H2O undergoes spin crossover (SCO) with a T1/2 of 309 K, slightly above room temperature. A literature survey of analogous trans-[FeII(Rdpt)2(NCX)2]-type complexes (53 distinct crystal structures) shows that for the complexes that are SCO active in the solid state the Fe–N≡C(X) angle is usually close to straight, 162–178°, whereas it is usually lower, 142–159°, for the complexes that remain HS. UV–vis studies in CHCl3 solution show that in each case the use of a 6:1 ratio of tolpyph/Fe(II) is required to ensure the iron(II) is present in solution as [FeII(tolpyph)2(NCE)2]. Interestingly, using this ratio, all three compounds are SCO-active in CDCl3 solution–in dramatic contrast to the solid-state findings. Specifically, while compounds 1 and 2 are not SCO-active in the solid state (they remain HS), they undergo gradual SCO in CDCl3 solution, with T1/2 values of 290 and 310 K, respectively. In CDCl3 solution, compound 3 has a T1/2 value of 288 K, which is 21 K lower than in the solid state. These results highlight the differences between solid state (ligand field; crystal packing) and solution (ligand field; solvation) effects on SCO, with the latter studies revealing room-temperature SCO for all three of these complexes.
中文翻译:

固溶对溶液自旋交叉和Fe–N≡C(X)角的重要性
通过[Fe II(吡啶)4(NCE)2 ]与1:2反应形成一个新的单核[Fe II(Rdpt)2(NCE)2 ]络合物(E = S,Se或BH 3)。单位吡啶基三唑配体4-(4-甲基苯基)-3-(2-吡啶基)-5-苯基-4 H -1,2,4-三唑(tolpyph)。获得三种复合物,形成六种不同的溶剂化形态:[Fe II(tolpyph)2(NCS)2 ]·H 2 O(1 ·H 2 O),1 ·1.5CH 3OH·0.5H 2 O,的[Fe II(tolpyph)2(NCSE)2 ](2),2 ·1.5H 2 O,的[Fe II(tolpyph)2(NCBH 3)2 ](3),和3 · ħ 2 O.单晶X-射线衍射表明,1 ·1.5CH 3 OH·0.5H 2 O和2是高自旋(HS)在100 K,而3是低自旋(LS)在100K和HS在373K。化合物3是具有NCBH 3共配体的[Fe II(Rdpt)2(NCE)2 ]型配合物的第一个结构特征实例:晶体堆积主要由芳族堆积相互作用决定。固态磁测量表明,低至50 K时1 ·H 2 O和2 ·1.5H 2 O仍保持HS,而3 ·H 2 O经历自旋交叉(SCO),T 1/2为309 K,略高于室内温度。的类似的文献调查的反式的[Fe - II(Rdpt)2(NCX)2 ]型配合物(53种不同的晶体结构)表明,对于在固态中具有SCO活性的配合物,Fe–N≡C(X)角通常接近笔直的162–178°,而对于仍为HS的配合物,通常较低,为142–159°。在CHCl 3溶液中的UV-vis研究表明,在每种情况下,都需要使用6:1的tolpyph / Fe(II)比例,以确保铁(II)以[Fe II(tolpyph)2( NCE)2 ]。有趣的是,使用该比率,这三种化合物在CDCl 3溶液中均具有SCO活性,这与固态研究结果形成了鲜明的对比。具体来说,化合物1和2在固态中不具有SCO活性(它们保持为HS),它们在CDCl 3溶液中经历逐渐的SCO ,T 1/2值分别为290和310K。在CDCl 3溶液中,化合物3的T 1/2值为288 K,比固态低21K。这些结果突出了固态(配体场;晶体堆积)和溶液(配体场;溶剂化)对SCO的影响之间的差异,后一项研究揭示了这三种配合物在室温下的SCO。
更新日期:2017-11-07
中文翻译:

固溶对溶液自旋交叉和Fe–N≡C(X)角的重要性
通过[Fe II(吡啶)4(NCE)2 ]与1:2反应形成一个新的单核[Fe II(Rdpt)2(NCE)2 ]络合物(E = S,Se或BH 3)。单位吡啶基三唑配体4-(4-甲基苯基)-3-(2-吡啶基)-5-苯基-4 H -1,2,4-三唑(tolpyph)。获得三种复合物,形成六种不同的溶剂化形态:[Fe II(tolpyph)2(NCS)2 ]·H 2 O(1 ·H 2 O),1 ·1.5CH 3OH·0.5H 2 O,的[Fe II(tolpyph)2(NCSE)2 ](2),2 ·1.5H 2 O,的[Fe II(tolpyph)2(NCBH 3)2 ](3),和3 · ħ 2 O.单晶X-射线衍射表明,1 ·1.5CH 3 OH·0.5H 2 O和2是高自旋(HS)在100 K,而3是低自旋(LS)在100K和HS在373K。化合物3是具有NCBH 3共配体的[Fe II(Rdpt)2(NCE)2 ]型配合物的第一个结构特征实例:晶体堆积主要由芳族堆积相互作用决定。固态磁测量表明,低至50 K时1 ·H 2 O和2 ·1.5H 2 O仍保持HS,而3 ·H 2 O经历自旋交叉(SCO),T 1/2为309 K,略高于室内温度。的类似的文献调查的反式的[Fe - II(Rdpt)2(NCX)2 ]型配合物(53种不同的晶体结构)表明,对于在固态中具有SCO活性的配合物,Fe–N≡C(X)角通常接近笔直的162–178°,而对于仍为HS的配合物,通常较低,为142–159°。在CHCl 3溶液中的UV-vis研究表明,在每种情况下,都需要使用6:1的tolpyph / Fe(II)比例,以确保铁(II)以[Fe II(tolpyph)2( NCE)2 ]。有趣的是,使用该比率,这三种化合物在CDCl 3溶液中均具有SCO活性,这与固态研究结果形成了鲜明的对比。具体来说,化合物1和2在固态中不具有SCO活性(它们保持为HS),它们在CDCl 3溶液中经历逐渐的SCO ,T 1/2值分别为290和310K。在CDCl 3溶液中,化合物3的T 1/2值为288 K,比固态低21K。这些结果突出了固态(配体场;晶体堆积)和溶液(配体场;溶剂化)对SCO的影响之间的差异,后一项研究揭示了这三种配合物在室温下的SCO。









































 京公网安备 11010802027423号
京公网安备 11010802027423号