当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iron(II) Complexes of a Hemilabile SNS Amido Ligand: Synthesis, Characterization, and Reactivity
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01802 Uttam K. Das 1 , Stephanie L. Daifuku 2 , Theresa E. Iannuzzi 2 , Serge I. Gorelsky 1 , Ilia Korobkov 1 , Bulat Gabidullin 1 , Michael L. Neidig 2 , R. Tom Baker 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01802 Uttam K. Das 1 , Stephanie L. Daifuku 2 , Theresa E. Iannuzzi 2 , Serge I. Gorelsky 1 , Ilia Korobkov 1 , Bulat Gabidullin 1 , Michael L. Neidig 2 , R. Tom Baker 1
Affiliation
|
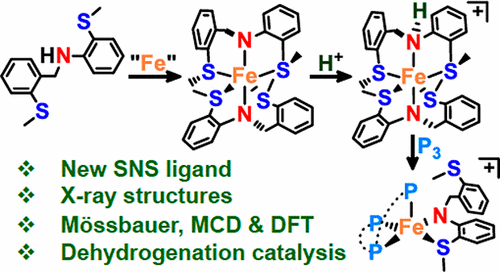
We report an easily prepared bis(thioether) amine ligand, SMeNHSMe, along with the synthesis, characterization, and reactivity of the paramagnetic iron(II) bis(amido) complex, [Fe(κ3-SMeNSMe)2] (1). Binding of the two different thioethers to Fe generates both five- and six-membered rings with Fe–S bonds in the five-membered rings (av 2.54 Å) being significantly shorter than those in the six-membered rings (av 2.71 Å), suggesting hemilability of the latter thioethers. Consistent with this hypothesis, magnetic circular dichroism (MCD) and computational (TD-DFT) studies indicate that 1 in solution contains a five-coordinate component [Fe(κ3-SMeNSMe)(κ2-SMeNSMe)] (2). This ligand hemilability was demonstrated further by reactivity studies of 1 with 2,2′-bipyridine, 1,2-bis(dimethylphosphino)ethane, and 2,6-dimethylphenyl isonitrile to afford iron(II) complexes [L2Fe(κ2-SMeNSMe)2] (3–5). Addition of a Brønsted acid, HNTf2, to 1 produces the paramagnetic, iron(II) amine–amido cation, [Fe(κ3-SMeNSMe)(κ3-SMeNHSMe)](NTf2) (6; Tf = SO2CF3). Cation 6 readily undergoes amine ligand substitution by triphos, affording the 16e– complex [Fe(κ2-SMeNSMe)(κ3-triphos)](NTf2) (7; triphos = bis(2-diphenylphosphinoethyl)phenylphosphine). These complexes are characterized by elemental analysis; 1H NMR, Mössbauer, IR, and UV–vis spectroscopy; and single-crystal X-ray diffraction. Preliminary results of amine–borane dehydrogenation catalysis show complex 7 to be a selective and particularly robust precatalyst.
中文翻译:

半不稳定的SNS酰胺配体的铁(II)配合物:合成,表征和反应性。
我们报告一个容易地制备二(硫醚)胺配体,S我Ñ ħ小号我,与合成,表征沿,并且所述顺磁性铁的反应(II)双(酰氨基)络合物,[Fe(上κ 3 -S我NS我)2 ](1)。两种不同的硫醚与Fe的结合会生成五元环和六元环,其中五元环(av 2.54Å)中的Fe–S键比六元环(av 2.71Å)中的Fe-S键短得多,暗示了后者硫醚的半透明性。与该假设一致,磁圆二色性(MCD)和计算(TD-DFT)研究表明1在溶液含有五坐标分量的[Fe(κ 3 -S我NS我)(κ 2 -S我NS我)](2)。这种配位体hemilability进一步通过反应性研究表明1与2,2'-联吡啶,1,2-双(二甲基膦基)乙烷,和2,6-二甲基苯基异腈,得到铁(II)络合物[L 2的Fe(κ 2 -S Me NS Me)2 ](3 – 5)。将布朗斯台德酸HNTf 2加到1产生顺磁性,铁(II)的胺-酰胺基阳离子,的[Fe(κ 3 -S我NS我)(κ 3 -S我Ñ ħ小号我)](NTF 2)(6 ; TF = SO 2 CF 3) 。阳离子6容易经历胺配体由三磷酸,得16E替代-复杂的[Fe(κ 2 -S我NS我)(κ 3 -triphos)](NTF 2)(7; 三(Triphos)=双(2-二苯基膦基乙基)苯基膦)。这些配合物的特征在于元素分析。1 H NMR,Mössbauer,IR和UV-vis光谱;和单晶X射线衍射。胺-硼烷脱氢催化的初步结果表明,配合物7是一种选择性的且特别坚固的预催化剂。
更新日期:2017-11-07
中文翻译:

半不稳定的SNS酰胺配体的铁(II)配合物:合成,表征和反应性。
我们报告一个容易地制备二(硫醚)胺配体,S我Ñ ħ小号我,与合成,表征沿,并且所述顺磁性铁的反应(II)双(酰氨基)络合物,[Fe(上κ 3 -S我NS我)2 ](1)。两种不同的硫醚与Fe的结合会生成五元环和六元环,其中五元环(av 2.54Å)中的Fe–S键比六元环(av 2.71Å)中的Fe-S键短得多,暗示了后者硫醚的半透明性。与该假设一致,磁圆二色性(MCD)和计算(TD-DFT)研究表明1在溶液含有五坐标分量的[Fe(κ 3 -S我NS我)(κ 2 -S我NS我)](2)。这种配位体hemilability进一步通过反应性研究表明1与2,2'-联吡啶,1,2-双(二甲基膦基)乙烷,和2,6-二甲基苯基异腈,得到铁(II)络合物[L 2的Fe(κ 2 -S Me NS Me)2 ](3 – 5)。将布朗斯台德酸HNTf 2加到1产生顺磁性,铁(II)的胺-酰胺基阳离子,的[Fe(κ 3 -S我NS我)(κ 3 -S我Ñ ħ小号我)](NTF 2)(6 ; TF = SO 2 CF 3) 。阳离子6容易经历胺配体由三磷酸,得16E替代-复杂的[Fe(κ 2 -S我NS我)(κ 3 -triphos)](NTF 2)(7; 三(Triphos)=双(2-二苯基膦基乙基)苯基膦)。这些配合物的特征在于元素分析。1 H NMR,Mössbauer,IR和UV-vis光谱;和单晶X射线衍射。胺-硼烷脱氢催化的初步结果表明,配合物7是一种选择性的且特别坚固的预催化剂。







































 京公网安备 11010802027423号
京公网安备 11010802027423号