当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Surface Charge of Hyperbranched Polymers on Cytotoxicity, Dynamic Cellular Uptake and Localization, Hemotoxicity, and Pharmacokinetics in Mice
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00611 Liyu Chen 1 , Joshua D. Simpson 1 , Adrian V. Fuchs 1 , Barbara E. Rolfe , Kristofer J. Thurecht 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00611 Liyu Chen 1 , Joshua D. Simpson 1 , Adrian V. Fuchs 1 , Barbara E. Rolfe , Kristofer J. Thurecht 1
Affiliation
|
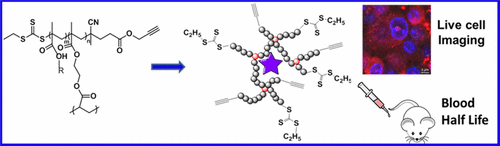
Nanoscaled polymeric materials are increasingly being investigated as pharmaceutical products, drug/gene delivery vectors, or health-monitoring devices. Surface charge is one of the dominant parameters that regulates nanomaterial behavior in vivo. In this paper, we demonstrated how control over chemical synthesis allowed manipulation of nanoparticle surface charge, which in turn greatly influenced the in vivo behavior. Three methacrylate/methacrylamide-based monomers were used to synthesize well-defined hyperbranched polymers (HBP) by reversible addition–fragmentation chain transfer (RAFT) polymerization. Each HBP had a hydrodynamic diameter of approximately 5 nm as determined by dynamic light scattering (DLS) and transmission electron microscopy (TEM). Incorporation of a fluorescent moiety within the polymeric nanoparticles allowed determination of how charge affected the in vivo pharmacokinetic behavior of the nanomaterials and the biological response to them. A direct correlation between surface charge, cellular uptake, and cytotoxicity was observed, with cationic HBPs exhibiting higher cellular uptake and cytotoxicity than their neutral and anionic counterparts. Evaluation of the distribution of the differently charged HBPs within macrophages showed that all HBPs accumulated in the cytoplasm, but cationic HBPs also trafficked to, and accumulated within, the nucleus. Although cationic HBPs caused slight hemolysis, this was generally below accepted levels for in vivo safety. Analysis of pharmacokinetic behavior showed that cationic and anionic HBPs had short blood half-lives of 1.82 ± 0.51 and 2.34 ± 0.93 h respectively, compared with 5.99 ± 2.30 h for neutral HBPs. This was attributed to the fact that positively charged surfaces are more readily covered with opsonin proteins and thus more visible to phagocytic cells. This was supported by in vitro flow cytometric and qualitative live cell imaging studies, which showed that cationic HBPs tended to be taken up by macrophages more effectively and rapidly than neutral and anionic particles.
中文翻译:

超支化聚合物表面电荷对小鼠细胞毒性,动态细胞摄取和定位,血液毒性和药代动力学的影响
越来越多地将纳米级聚合物材料作为药物产品,药物/基因传递载体或健康监测设备进行研究。表面电荷是调节体内纳米材料行为的主要参数之一。在本文中,我们证明了对化学合成的控制如何允许对纳米颗粒表面电荷的操纵,从而极大地影响了体内行为。通过可逆的加成-断裂链转移(RAFT)聚合反应,使用了三种基于甲基丙烯酸酯/甲基丙烯酰胺的单体来合成定义明确的超支化聚合物(HBP)。通过动态光散射(DLS)和透射电子显微镜(TEM)确定,每个HBP的流体力学直径约为5 nm。在聚合物纳米颗粒中掺入荧光部分可以确定电荷如何影响体内纳米材料的药代动力学行为及其生物学响应。观察到表面电荷,细胞摄取和细胞毒性之间存在直接相关性,阳离子型HBP的中性摄取和细胞毒性均高于其中性和阴离子对应物。巨噬细胞中带不同电荷的HBP分布的评估表明,所有HBP都积累在细胞质中,但阳离子HBP也贩运并积累在细胞核中。尽管阳离子型HBP引起轻微溶血,但通常低于体内可接受的水平安全。药代动力学行为分析表明,阳离子和阴离子型HBPs的血液半衰期分别为1.82±0.51和2.34±0.93 h,而中性HBPs的血液半衰期为5.99±2.30 h。这归因于这样的事实,带正电的表面更容易被调理素蛋白覆盖,因此对吞噬细胞更可见。体外流式细胞术和定性活细胞成像研究支持了这一点,该研究表明,与中性和阴离子颗粒相比,阳离子型HBP倾向于被巨噬细胞更有效,更快地吸收。
更新日期:2017-11-08
中文翻译:

超支化聚合物表面电荷对小鼠细胞毒性,动态细胞摄取和定位,血液毒性和药代动力学的影响
越来越多地将纳米级聚合物材料作为药物产品,药物/基因传递载体或健康监测设备进行研究。表面电荷是调节体内纳米材料行为的主要参数之一。在本文中,我们证明了对化学合成的控制如何允许对纳米颗粒表面电荷的操纵,从而极大地影响了体内行为。通过可逆的加成-断裂链转移(RAFT)聚合反应,使用了三种基于甲基丙烯酸酯/甲基丙烯酰胺的单体来合成定义明确的超支化聚合物(HBP)。通过动态光散射(DLS)和透射电子显微镜(TEM)确定,每个HBP的流体力学直径约为5 nm。在聚合物纳米颗粒中掺入荧光部分可以确定电荷如何影响体内纳米材料的药代动力学行为及其生物学响应。观察到表面电荷,细胞摄取和细胞毒性之间存在直接相关性,阳离子型HBP的中性摄取和细胞毒性均高于其中性和阴离子对应物。巨噬细胞中带不同电荷的HBP分布的评估表明,所有HBP都积累在细胞质中,但阳离子HBP也贩运并积累在细胞核中。尽管阳离子型HBP引起轻微溶血,但通常低于体内可接受的水平安全。药代动力学行为分析表明,阳离子和阴离子型HBPs的血液半衰期分别为1.82±0.51和2.34±0.93 h,而中性HBPs的血液半衰期为5.99±2.30 h。这归因于这样的事实,带正电的表面更容易被调理素蛋白覆盖,因此对吞噬细胞更可见。体外流式细胞术和定性活细胞成像研究支持了这一点,该研究表明,与中性和阴离子颗粒相比,阳离子型HBP倾向于被巨噬细胞更有效,更快地吸收。











































 京公网安备 11010802027423号
京公网安备 11010802027423号