当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of Highly Stereodefined Tetrasubstituted Acyclic All-Carbon Olefins via a Syn-Elimination Approach
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.orglett.7b03141 Ngiap-Kie Lim 1 , Patrick Weiss 1 , Beryl X. Li 1 , Christina H. McCulley 2 , Stephanie R. Hare 2 , Bronwyn L. Bensema 2 , Teresa A. Palazzo 2 , Dean J. Tantillo 2 , Haiming Zhang 1 , Francis Gosselin 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-08 00:00:00 , DOI: 10.1021/acs.orglett.7b03141 Ngiap-Kie Lim 1 , Patrick Weiss 1 , Beryl X. Li 1 , Christina H. McCulley 2 , Stephanie R. Hare 2 , Bronwyn L. Bensema 2 , Teresa A. Palazzo 2 , Dean J. Tantillo 2 , Haiming Zhang 1 , Francis Gosselin 1
Affiliation
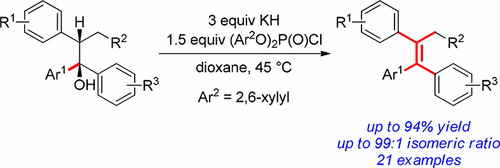
|
An efficient synthesis of stereodefined tetrasubstituted acyclic all-carbon olefins has been developed via a bis(2,6-xylyl)phosphate formation of stereoenriched tertiary alcohols, followed by in situ syn-elimination of the corresponding phosphates under mild conditions. This chemistry tolerates a wide variety of electronically and sterically diverse substrates and generates the desired tetrasubstituted olefins in high yields and stereoselectivities (>95:5) in most cases. This stereocontrolled olefin synthesis has been applied to the synthesis of anticancer drug tamoxifen in three steps from commercially available 1,2-diphenylbutan-1-one in 97:3 stereoselectivity and 78% overall yield.
中文翻译:

通过高度Stereodefined四取代非周期性全碳烯烃合成的Syn在剔除方法
通过形成双(2,6-二甲苯基)磷酸形成富含立体异构的叔醇,然后在温和条件下原位合成消除相应的磷酸酯,已经开发出有效合成立体确定的四取代的无环全碳烯烃的方法。在大多数情况下,这种化学方法可耐受多种电子和空间上不同的底物,并以高收率和立体选择性(> 95:5)生成所需的四取代烯烃。该立体控制的烯烃合成已经以三步法从97:3立体选择性和78%的总收率应用到抗癌药他莫昔芬的合成中,该步骤由市售的1,2-二苯基丁烷-1-酮合成。
更新日期:2017-11-08
中文翻译:

通过高度Stereodefined四取代非周期性全碳烯烃合成的Syn在剔除方法
通过形成双(2,6-二甲苯基)磷酸形成富含立体异构的叔醇,然后在温和条件下原位合成消除相应的磷酸酯,已经开发出有效合成立体确定的四取代的无环全碳烯烃的方法。在大多数情况下,这种化学方法可耐受多种电子和空间上不同的底物,并以高收率和立体选择性(> 95:5)生成所需的四取代烯烃。该立体控制的烯烃合成已经以三步法从97:3立体选择性和78%的总收率应用到抗癌药他莫昔芬的合成中,该步骤由市售的1,2-二苯基丁烷-1-酮合成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号