当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoredox Catalysis Induced Bisindolylation of Ethers/Alcohols via Sequential C–H and C–O Bond Cleavage
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03073 Lu Ye 1 , Sai-Hu Cai 1, 2 , Ding-Xing Wang 1 , Yi-Qiu Wang 1 , Lin-Jie Lai 1 , Chao Feng 1 , Teck-Peng Loh 2, 3
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.orglett.7b03073 Lu Ye 1 , Sai-Hu Cai 1, 2 , Ding-Xing Wang 1 , Yi-Qiu Wang 1 , Lin-Jie Lai 1 , Chao Feng 1 , Teck-Peng Loh 2, 3
Affiliation
|
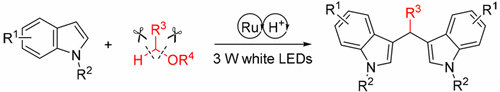
A visible-light-engaged 2-fold site-selective alkylation of indole derivatives with aliphatic ethers or alcohols has been accomplished for easy access to symmetric 3,3′-bisindolylmethane derivatives. The experimental data suggest a sequential photoredox catalysis induced radical addition and proton-mediated Friedel–Crafts alkylation mechanism.
中文翻译:

光氧化还原催化通过连续的C–H和C–O键裂解引起的醚/醇双双烯丙基化
为了容易获得对称的3,3′-二吲哚基甲烷衍生物,已经实现了吲哚衍生物与脂肪族醚或醇的可见光结合的2-位点选择性烷基化。实验数据表明,相继的光氧化还原催化诱导了自由基的加成和质子介导的Friedel-Crafts烷基化机理。
更新日期:2017-11-07
中文翻译:

光氧化还原催化通过连续的C–H和C–O键裂解引起的醚/醇双双烯丙基化
为了容易获得对称的3,3′-二吲哚基甲烷衍生物,已经实现了吲哚衍生物与脂肪族醚或醇的可见光结合的2-位点选择性烷基化。实验数据表明,相继的光氧化还原催化诱导了自由基的加成和质子介导的Friedel-Crafts烷基化机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号