当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Is C3N4 Chemically Stable toward Reactive Oxygen Species in Sunlight-Driven Water Treatment?
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.est.7b04215 Jiadong Xiao 1, 2 , Qingzhen Han 1 , Yongbing Xie 1 , Jin Yang 1 , Qiaozhi Su 1 , Yue Chen 1 , Hongbin Cao 1
Environmental Science & Technology ( IF 11.4 ) Pub Date : 2017-11-07 00:00:00 , DOI: 10.1021/acs.est.7b04215 Jiadong Xiao 1, 2 , Qingzhen Han 1 , Yongbing Xie 1 , Jin Yang 1 , Qiaozhi Su 1 , Yue Chen 1 , Hongbin Cao 1
Affiliation
|
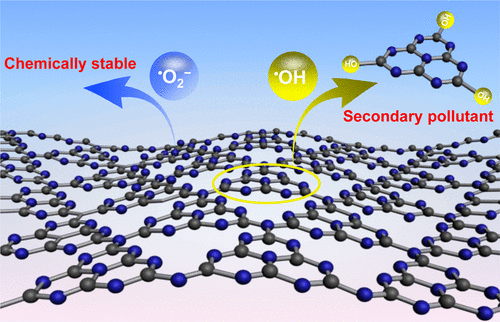
Reactive oxygen species (ROS) are key oxidants for the degradation of organic pollutants in sunlight-driven photocatalytic water treatment, but their interaction with the photocatalyst is easily ignored and, hence, is comparatively poorly understood. Here we show that graphitic carbon nitride (C3N4, a famous visible-light-responsive photocatalyst) is chemically stable toward ozone and superoxide radical; in contrast, hydroxyl radical (•OH) can tear the heptazine unit directly from C3N4 to form cyameluric acid and further release nitrates into the aqueous environment. The ratios of released nitrogen from nanosheet-structured C3N4 and bulk C3N4 that finally exists in the form of NO3– reach 9.5 and 6.8 mol % in initially ultrapure water, respectively, after 10 h treatment by solar photocatalytic ozonation, which can rapidly generate abundant •OH to attack C3N4. On a positive note, in the presence of organic pollutants which compete against C3N4 for •OH, the C3N4 decomposition has been completely or partially blocked; therefore, the stability of C3N4 under practical working conditions has been obviously preserved. This work supplements the missing knowledge of the chemical instability of C3N4 toward •OH and calls for attention to the potential deactivation and secondary pollution of catalysts in •OH-involved water treatment processes.
中文翻译:

在阳光驱动的水处理中,C 3 N 4对化学活性氧是否稳定?
活性氧(ROS)是在阳光驱动的光催化水处理过程中降解有机污染物的关键氧化剂,但是它们与光催化剂的相互作用很容易被忽略,因此对它的理解相对较差。在这里,我们显示了石墨碳氮化物(C 3 N 4,一种著名的可见光响应型光催化剂)对臭氧和超氧自由基的化学稳定性。相反,羟自由基(• OH)可以直接从C 3 N 4撕开庚嗪单元,形成环戊二酸,并进一步将硝酸盐释放到水性环境中。纳米片结构的C 3 N 4和本体C 3释放的氮的比率Ñ 4终于存在NO的形式3 -达到9.5和6.8%(摩尔)在最初的超纯水,分别由后光催化太阳能臭氧化10小时处理,其可以快速地产生丰富• OH攻击ç 3 Ñ 4。在积极的,在这种竞争向C的有机污染物的存在3 Ñ 4为• OH中,C 3 Ñ 4分解已被完全或部分封端; 因此,C 3 N 4的稳定性在实际的工作条件下得到了明显的保留。这项工作补充了对C 3 N 4对• OH的化学不稳定性的认识,并呼吁关注•涉及OH的水处理过程中催化剂的潜在失活和二次污染。
更新日期:2017-11-07
中文翻译:

在阳光驱动的水处理中,C 3 N 4对化学活性氧是否稳定?
活性氧(ROS)是在阳光驱动的光催化水处理过程中降解有机污染物的关键氧化剂,但是它们与光催化剂的相互作用很容易被忽略,因此对它的理解相对较差。在这里,我们显示了石墨碳氮化物(C 3 N 4,一种著名的可见光响应型光催化剂)对臭氧和超氧自由基的化学稳定性。相反,羟自由基(• OH)可以直接从C 3 N 4撕开庚嗪单元,形成环戊二酸,并进一步将硝酸盐释放到水性环境中。纳米片结构的C 3 N 4和本体C 3释放的氮的比率Ñ 4终于存在NO的形式3 -达到9.5和6.8%(摩尔)在最初的超纯水,分别由后光催化太阳能臭氧化10小时处理,其可以快速地产生丰富• OH攻击ç 3 Ñ 4。在积极的,在这种竞争向C的有机污染物的存在3 Ñ 4为• OH中,C 3 Ñ 4分解已被完全或部分封端; 因此,C 3 N 4的稳定性在实际的工作条件下得到了明显的保留。这项工作补充了对C 3 N 4对• OH的化学不稳定性的认识,并呼吁关注•涉及OH的水处理过程中催化剂的潜在失活和二次污染。



























 京公网安备 11010802027423号
京公网安备 11010802027423号