JAMA Pediatrics ( IF 24.7 ) Pub Date : 2017-11-01 , DOI: 10.1001/jamapediatrics.2017.2007 Sybil G. Hosek 1 , Raphael J. Landovitz 2 , Bill Kapogiannis 3 , George K. Siberry 3 , Bret Rudy 4 , Brandy Rutledge 5 , Nancy Liu 5 , D. Robert Harris 5 , Kathleen Mulligan 6 , Gregory Zimet 7 , Kenneth H. Mayer 8 , Peter Anderson 9 , Jennifer J. Kiser 9 , Michelle Lally 10 , Jennifer Brothers 1 , Kelly Bojan 11 , Jim Rooney 12 , Craig M. Wilson 13
|
Importance Adolescents represent a key population for implementing preexposure prophylaxis (PrEP) interventions worldwide, yet tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) for PrEP is only licensed for adults.
Objective To examine the safety of and adherence to PrEP along with changes in sexual risk behavior among adolescent men who have sex with men (MSM).
Design, Setting, and Participants Adolescent Medicine Trials Network for HIV/AIDS Interventions 113 (Project PrEPare) was a PrEP demonstration project that evaluated the safety, tolerability, and acceptability of TDF/FTC and patterns of use, rates of adherence, and patterns of sexual risk behavior among healthy young MSM aged 15 to 17 years. Participants were recruited from adolescent medicine clinics and their community partners in 6 US cities, had negative test results for human immunodeficiency virus (HIV) but were at high risk for acquiring an infection, and were willing to participate in a behavioral intervention and accept TDF/FTC as PrEP.
Exposures All participants completed an individualized evidence-based behavioral intervention and were provided with daily TDF/FTC as PrEP for 48 weeks.
Main Outcomes and Measures The main objectives were to: (1) provide additional safety data regarding TDF/FTC use among young MSM who had negative test results for HIV; (2) examine the acceptability, patterns of use, rates of adherence, and measured levels of tenofovir diphosphate in dried blood spots; and (3) examine patterns of risk behavior when young MSM were provided with a behavioral intervention in conjunction with open-label TDF/FTC.
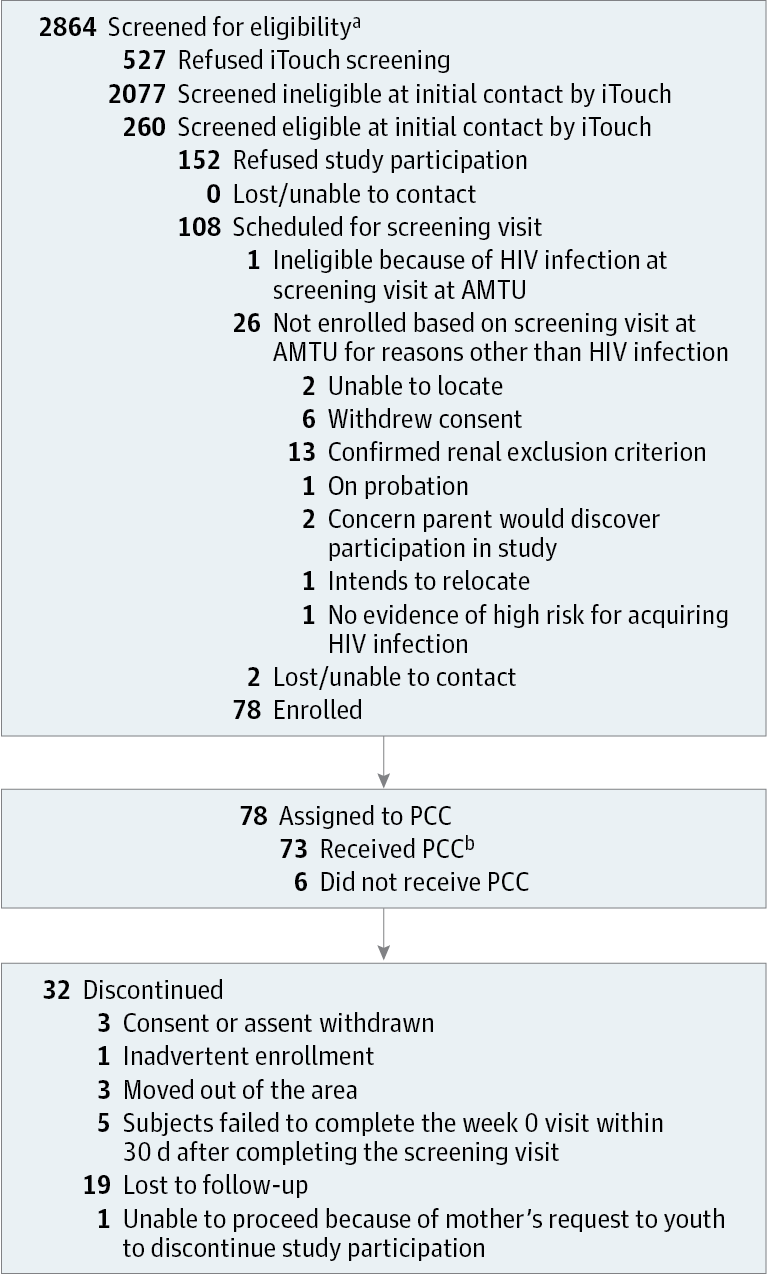
Results Among 2864 individuals screened (from August 2013 to September 2014), 260 were eligible and 78 were enrolled (mean [SD] age, 16.5 [0.73] years), of whom 2 (3%) were Asian/Pacific Islander, 23 (29%) were black/African American, 11 (14%) were white, 16 (21%) were white Hispanic, and 26 (33%) were other/mixed race/ethnicity. Over 48 weeks of PrEP use, 23 sexually transmitted infections were diagnosed in 12 participants. The HIV seroconversion rate was 6.4 (95% CI: 1.3-18.7) per 100 person-years. Tenofovir diphosphate levels consistent with a high degree of anti-HIV protection (>700 fmol/punch) were found in 42 (54%), 37 (47%), 38 (49%), 22 (28%), 13 (17%), and 17 (22%) participants at weeks 4, 8, 12, 24, 36, and 48, respectively.
Conclusions and Relevance Adolescent Medicine Trials Network for HIV/AIDS Interventions 113 enrolled a diverse sample of adolescent MSM at risk for HIV who consented to study participation. Approximately half achieved protective drug levels during the monthly visits, but adherence decreased with quarterly visits. Youth may need additional contact with clinical staff members to maintain high adherence.
Trial Registration clinicaltrials.gov Identifier: NCT01769456
中文翻译:

在美国与15至17岁男性发生性行为的青春期男性进行抗逆转录病毒前暴露预防的安全性和可行性
重要性 青少年是全世界实施暴露前预防(PrEP)干预措施的关键人群,但替诺福韦富马酸替诺福韦酯/曲美他滨(TDF / FTC)仅适用于成人。
目的 探讨与男性发生性关系(MSM)的青春期男性中PrEP的安全性和依从性,以及性风险行为的变化。
设计,设置和参与者 针对HIV / AIDS干预的青少年医学试验网络113(PrEPare项目)是PrEP演示项目,评估了TDF / FTC的安全性,耐受性和可接受性以及使用模式,依从率和使用方式15至17岁的健康年轻MSM中的性风险行为。参与者是从美国6个城市的青少年医学诊所及其社区合作伙伴中招募的,他们的人类免疫缺陷病毒(HIV)测试结果阴性,但感染风险很高,并且愿意参加行为干预并接受TDF / FTC作为PrEP。
暴露 所有参与者均完成了基于证据的个性化行为干预,并每天接受48周的TDF / FTC作为PrEP。
主要结果和措施 主要目标是:(1)提供有关HIV检测结果阴性的年轻MSM中有关TDF / FTC使用的附加安全性数据;(2)检查干血斑中替诺福韦二磷酸替诺福韦的可接受性,使用方式,依从率和测量水平;(3)研究在向年轻MSM进行行为干预并结合开放标签TDF / FTC时的风险行为模式。
结果 在筛选的2864人中(从2013年8月至2014年9月),有260名符合条件,并入组了78名(平均[SD]年龄为16.5 [0.73]岁),其中2(3%)为亚太岛民,23(黑人/非裔美国人占29%,白人/黑人占11%(14%),西班牙裔白人占16%(21%),其他/混合种族/族裔占26%(33%)。使用PrEP超过48周,在12名参与者中诊断出23种性传播感染。每100人年的HIV血清转化率为6.4(95%CI:1.3-18.7)。在42(54%),37(47%),38(49%),22(28%),13(17)中发现替诺福韦二磷酸水平与高度抗HIV保护(> 700 fmol /打孔)相一致。 %)和分别在第4、8、12、24、36和48周的17位(22%)参与者。
结论和相关性 干预艾滋病毒/艾滋病的青少年医学试验网络113纳入了接受研究参与的,有感染艾滋病毒风险的青少年MSM的各种样本。大约一半的患者在每月的就诊期间达到了保护性药物水平,但是依从性随季度的就诊而下降。青年可能需要与临床工作人员进行更多接触,以保持较高的依从性。
试验注册 临床试验.gov标识符:NCT01769456











































 京公网安备 11010802027423号
京公网安备 11010802027423号