当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Asymmetric Induction in Proline-Catalyzed Aldol Reactions: Reagent-Controlled Addition Reactions of 2,2-Dimethyl-1,3-dioxane-5-one to Acyclic Chiral α-Branched Aldehydes
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-11-06 04:40:25 , DOI: 10.1002/ejoc.201701073 Jasna Marjanovic Trajkovic 1 , Vesna Milanovic 2 , Zorana Ferjancic 2 , Radomir N. Saicic 2, 3
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2017-11-06 04:40:25 , DOI: 10.1002/ejoc.201701073 Jasna Marjanovic Trajkovic 1 , Vesna Milanovic 2 , Zorana Ferjancic 2 , Radomir N. Saicic 2, 3
Affiliation
|
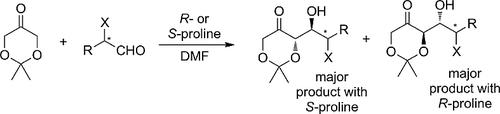
Proline-catalyzed aldol addition reactions of 2,2-dimethyl-1,3-dioxane-5-one to chiral aldehydes proceed under reagent stereocontrol (in both matched and mismatched cases) if aldehyde α-oxy or α-amino substituents are acyclic. For cyclic substituents, the stereochemical outcome of the aldolization in mismatched cases is difficult to predict.
中文翻译:

脯氨酸催化的醛醇缩合反应的不对称诱导:2,2-二甲基-1,3-二恶烷-5-酮与无环手性α-支化醛的试剂控制加成反应
如果醛的α-氧基或α-氨基取代基是无环的,则在试剂立体控制下(在匹配和不匹配的情况下),脯氨酸催化的2,2-二甲基-1,3-二恶烷-5-酮与手性醛的羟醛加成反应。对于环状取代基,在不匹配的情况下醛醇缩合的立体化学结果很难预测。
更新日期:2017-11-06
中文翻译:

脯氨酸催化的醛醇缩合反应的不对称诱导:2,2-二甲基-1,3-二恶烷-5-酮与无环手性α-支化醛的试剂控制加成反应
如果醛的α-氧基或α-氨基取代基是无环的,则在试剂立体控制下(在匹配和不匹配的情况下),脯氨酸催化的2,2-二甲基-1,3-二恶烷-5-酮与手性醛的羟醛加成反应。对于环状取代基,在不匹配的情况下醛醇缩合的立体化学结果很难预测。











































 京公网安备 11010802027423号
京公网安备 11010802027423号