European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-11-04 , DOI: 10.1016/j.ejmech.2017.11.005 Beata Żołnowska , Jarosław Sławiński , Krzysztof Szafrański , Andrea Angeli , Claudiu T. Supuran , Anna Kawiak , Miłosz Wieczór , Joanna Zielińska , Tomasz Bączek , Sylwia Bartoszewska
|
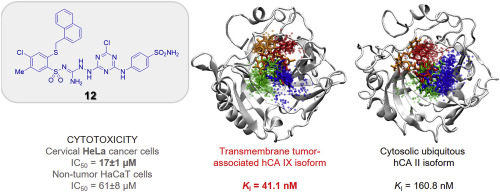
A series of novel 2-(2-arylmethylthio-4-chloro-5-methylbenzenesulfonyl)-1-(6-substituted-4-chloro-1,3,5-triazin-2-ylamino)guanidine derivatives 9–20 have been synthesized by substitution of chlorine atom at the 1,3,5-triazine ring in compounds 5–8 with 3- or 4-aminobenzenesulfonamide and 4-(aminomethyl)benzenesulfonamide hydrochloride. All the synthesized compounds were evaluated for their inhibitory activity toward hCA I, II, IX and XII as well as anticancer activity against HeLa, HCT-116 and MCF-7 human tumor cell lines. The investigated compounds showed weak inhibitory potency against the human CA I, while activity toward hCA II was differentiated and depended on structure of inhibitor (KI: 5.4–933.1 nM). Compounds containing the 4-sulfamoylphenyl moiety (9–12) exhibited the strongest inhibitory activity against hCA IX with KI values from 37.1 to 42.9 nM, as well as against hCA XII in range of 31–91.9 nM. The most promising compound 12 (KI = 41 nM) showed the highest selectivity toward hCA IX versus hCA I (hCA I/hCA IX = 18) and hCA II (hCA II/hCA IX = 4). Compound 12 displayed prominent cytotoxic effect selectively toward HeLa cancer cells (IC50 = 17 μM) and did not exhibit toxicity to the non-cancerous HaCaT cells. In silico analysis suggested that despite the lack of a single binding pose, the selective affinity is conferred by specific interactions with an arginine moiety, as well as better-defined binding modes within the active site.
中文翻译:

新型2-(2-芳基甲硫基-4-氯-5-甲基苯磺酰基)-1-(1,3,5-三嗪-2-基氨基)胍衍生物:抑制人碳酸酐酶胞质同工酶I和II和跨膜肿瘤-相关的同工酶IX和XII,抗癌活性和分子模型研究
一系列的新的2-(2- arylmethylthio -4-氯-5-甲基苯磺酰基)-1-(6-取代-4-氯-1,3,5-三嗪-2-基氨基)胍衍生物9-20已通过用3-或4-氨基苯磺酰胺和4-(氨基甲基)苯磺酰胺盐酸盐取代化合物5-8中的1,3,5-三嗪环上的氯原子合成。评价所有合成的化合物对hCA I,II,IX和XII的抑制活性以及对HeLa,HCT-116和MCF-7人肿瘤细胞系的抗癌活性。所研究的化合物对人CA I的抑制力较弱,而对hCA II的活性则有所差异,并取决于抑制剂的结构(K I:5.4–933.1 nM)。含有4-氨磺酰苯基部分(化合物9 - 12)表现出对HCA IX具有最强抑制活性ķ我值从37.1至42.9纳米,以及对在31-91.9纳米的范围HCA XII。最有前途的化合物12(ķ我 = 41 nM)的表现出对HCA IX与HCA的最高选择性I(HCA I / HCA IX = 18)和HCA II(HCA II / HCA IX = 4)。化合物12对HeLa癌细胞(IC 50 = 17μM)选择性地表现出显着的细胞毒性作用,而对非癌的HaCaT细胞没有毒性。在计算机上 分析表明,尽管缺乏单个结合姿势,但选择性结合是通过与精氨酸部分的特异性相互作用以及在活性位点内更明确定义的结合模式而赋予的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号