当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Coadministered Water on the In Vivo Performance of Oral Formulations Containing N-Acetylcysteine: An In Vitro Approach Using the Dynamic Open Flow-Through Test Apparatus
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00763 Maximilian Sager 1 , Felix Schneider 1 , Philipp Jedamzik 1 , Markus Wiedmann 2 , Ellen Schremmer 2 , Mirko Koziolek 1 , Werner Weitschies 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00763 Maximilian Sager 1 , Felix Schneider 1 , Philipp Jedamzik 1 , Markus Wiedmann 2 , Ellen Schremmer 2 , Mirko Koziolek 1 , Werner Weitschies 1
Affiliation
|
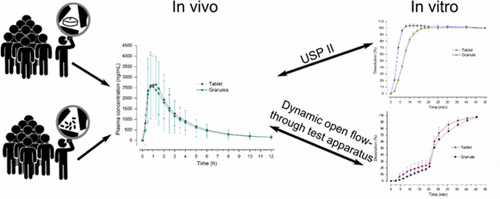
The drug plasma profile after oral administration of immediate release dosage forms can be affected by the human gastrointestinal physiology, the formulation, and the drug itself. In this work, we investigated the in vivo and in vitro performance of two formulations (granules vs. tablet) containing the highly soluble drug N-Acetylcysteine (BCS class I). Thereby, special attention was paid to the effect of the dosage form and the coadministration of water on drug release. Interestingly, the in vivo results from a pharmacokinetic study with 11 healthy volunteers indicated that the drug plasma concentrations were comparable for the tablet given with water as well as for the granules given with and without water. In order to mechanistically understand this outcome, we used a biorelevant dissolution test device, the dynamic open flow-through test apparatus. With the aid of this test apparatus, we were able to simulate biorelevant parameters, such as gastric emptying, hydrodynamic flow as well as physical stress. By this, it was possible to mimic the intake conditions of the clinical trial (i.e., drug intake with and without water). Whereas the experiments in the USP paddle apparatus revealed differences between the two formulations, we could not observe significant differences in the release profiles of the two formulations by using the dynamic open flow-through test apparatus. Even by considering the different intake conditions, drug release was slow and amounted to around 30% until simulated gastric emptying. These results suggest that dissolution was irrespective of coadministered water and the formulation. Despite the high aqueous solubility of N-Acetylcysteine, the limiting factor for drug release was the slow dissolution rate in relation to the gastric emptying rate under simulated gastric conditions. Thus, in case of administration together with water, large amounts of the drug are still present in the stomach even after complete gastric emptying of the water. Consequently, the absorption of the drug is largely controlled by the nature of gastric emptying of the remaining drug. The data of this study indicated that the water emptying kinetics are only determining drug absorption if drug release is rapid enough. If this is not the case, physiological mechanisms, such as the migrating motor complex, play an important role for oral drug delivery.
中文翻译:

共同施用的水对含N-乙酰半胱氨酸的口服制剂体内性能的影响:使用动态开放式流通测试仪的体外方法
口服给药速释剂型后的药物血浆分布可能受到人体胃肠道生理,制剂和药物本身的影响。在这项工作中,我们研究了含有高度可溶药物N-乙酰半胱氨酸(BCS I类)的两种制剂(颗粒与片剂)的体内和体外性能。因此,应特别注意剂型和水的共同给药对药物释放的影响。有趣的是,体内一项针对11名健康志愿者进行的药代动力学研究的结果表明,与水一起服用的片剂以及有水和无水的颗粒剂相比,药物血浆浓度是可比的。为了从机械角度理解此结果,我们使用了生物相关的溶出度测试设备,即动态开放式流通测试设备。借助该测试仪器,我们能够模拟与生物有关的参数,例如胃排空,流体动力流动以及身体压力。这样,可以模拟临床试验的摄入条件(即,有水和无水的药物摄入)。尽管在USP桨式装置中进行的实验揭示了两种配方之间的差异,通过使用动态开放式流通测试设备,我们无法观察到两种制剂的释放曲线有显着差异。即使考虑到不同的摄入条件,药物释放也很缓慢,直到模拟胃排空之前,药物释放量约为30%。这些结果表明溶出与水和制剂的共同使用无关。尽管N-乙酰半胱氨酸具有很高的水溶性,但在模拟的胃条件下,药物释放的限制因素是相对于胃排空速率而言缓慢的溶出速率。因此,在与水一起给药的情况下,即使在胃完全排空水之后,胃中仍存在大量药物。所以,药物的吸收在很大程度上受剩余药物胃排空的性质控制。这项研究的数据表明,如果药物释放足够快,则排空动力学仅决定药物的吸收。如果不是这种情况,则生理机制,例如迁移的运动复合物,对于口服药物递送起着重要作用。
更新日期:2017-11-05
中文翻译:

共同施用的水对含N-乙酰半胱氨酸的口服制剂体内性能的影响:使用动态开放式流通测试仪的体外方法
口服给药速释剂型后的药物血浆分布可能受到人体胃肠道生理,制剂和药物本身的影响。在这项工作中,我们研究了含有高度可溶药物N-乙酰半胱氨酸(BCS I类)的两种制剂(颗粒与片剂)的体内和体外性能。因此,应特别注意剂型和水的共同给药对药物释放的影响。有趣的是,体内一项针对11名健康志愿者进行的药代动力学研究的结果表明,与水一起服用的片剂以及有水和无水的颗粒剂相比,药物血浆浓度是可比的。为了从机械角度理解此结果,我们使用了生物相关的溶出度测试设备,即动态开放式流通测试设备。借助该测试仪器,我们能够模拟与生物有关的参数,例如胃排空,流体动力流动以及身体压力。这样,可以模拟临床试验的摄入条件(即,有水和无水的药物摄入)。尽管在USP桨式装置中进行的实验揭示了两种配方之间的差异,通过使用动态开放式流通测试设备,我们无法观察到两种制剂的释放曲线有显着差异。即使考虑到不同的摄入条件,药物释放也很缓慢,直到模拟胃排空之前,药物释放量约为30%。这些结果表明溶出与水和制剂的共同使用无关。尽管N-乙酰半胱氨酸具有很高的水溶性,但在模拟的胃条件下,药物释放的限制因素是相对于胃排空速率而言缓慢的溶出速率。因此,在与水一起给药的情况下,即使在胃完全排空水之后,胃中仍存在大量药物。所以,药物的吸收在很大程度上受剩余药物胃排空的性质控制。这项研究的数据表明,如果药物释放足够快,则排空动力学仅决定药物的吸收。如果不是这种情况,则生理机制,例如迁移的运动复合物,对于口服药物递送起着重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号