当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overcoming double-step CO2 adsorption and minimizing water co-adsorption in bulky diamine-appended variants of Mg2(dobpdc)
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7sc04266c Phillip J. Milner 1, 2, 3, 4 , Jeffrey D. Martell 1, 2, 3, 4 , Rebecca L. Siegelman 1, 2, 3, 4 , David Gygi 4, 5, 6, 7 , Simon C. Weston 4, 8, 9, 10 , Jeffrey R. Long 1, 2, 3, 4, 11
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-26 00:00:00 , DOI: 10.1039/c7sc04266c Phillip J. Milner 1, 2, 3, 4 , Jeffrey D. Martell 1, 2, 3, 4 , Rebecca L. Siegelman 1, 2, 3, 4 , David Gygi 4, 5, 6, 7 , Simon C. Weston 4, 8, 9, 10 , Jeffrey R. Long 1, 2, 3, 4, 11
Affiliation
|
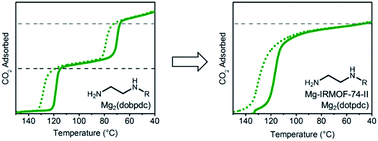
Alkyldiamine-functionalized variants of the metal–organic framework Mg2(dobpdc) (dobpdc4− = 4,4′-dioxidobiphenyl-3,3′-dicarboxylate) are promising for CO2 capture applications owing to their unique step-shaped CO2 adsorption profiles resulting from the cooperative formation of ammonium carbamate chains. Primary,secondary (1°,2°) alkylethylenediamine-appended variants are of particular interest because of their low CO2 step pressures (≤1 mbar at 40 °C), minimal adsorption/desorption hysteresis, and high thermal stability. Herein, we demonstrate that further increasing the size of the alkyl group on the secondary amine affords enhanced stability against diamine volatilization, but also leads to surprising two-step CO2 adsorption/desorption profiles. This two-step behavior likely results from steric interactions between ammonium carbamate chains induced by the asymmetrical hexagonal pores of Mg2(dobpdc) and leads to decreased CO2 working capacities and increased water co-adsorption under humid conditions. To minimize these unfavorable steric interactions, we targeted diamine-appended variants of the isoreticularly expanded framework Mg2(dotpdc) (dotpdc4− = 4,4′′-dioxido-[1,1′:4′,1′′-terphenyl]-3,3′′-dicarboxylate), reported here for the first time, and the previously reported isomeric framework Mg-IRMOF-74-II or Mg2(pc-dobpdc) (pc-dobpdc4− = 3,3′-dioxidobiphenyl-4,4′-dicarboxylate, pc = para-carboxylate), which, in contrast to Mg2(dobpdc), possesses uniformally hexagonal pores. By minimizing the steric interactions between ammonium carbamate chains, these frameworks enable a single CO2 adsorption/desorption step in all cases, as well as decreased water co-adsorption and increased stability to diamine loss. Functionalization of Mg2(pc-dobpdc) with large diamines such as N-(n-heptyl)ethylenediamine results in optimal adsorption behavior, highlighting the advantage of tuning both the pore shape and the diamine size for the development of new adsorbents for carbon capture applications.
中文翻译:

在Mg 2(dobpdc)的笨重的二胺附加变体中,克服了CO 2的两步吸附并最大程度地减少了水的共吸附
金属-有机骨架Mg 2(dobpdc)(dobpdc 4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate)的烷基二胺官能化变体因其独特的阶梯状CO 2而有望用于CO 2捕集应用。氨基甲酸铵链协同形成的吸附曲线。伯,仲(1°,2°)烷基乙二胺附加变体因其CO 2低而特别受关注阶跃压力(在40°C时≤1mbar),最小的吸附/解吸滞后和高热稳定性。在本文中,我们证明了进一步增加仲胺上烷基的尺寸提供了增强的抵抗二胺挥发的稳定性,而且还导致了令人惊讶的两步式CO 2吸附/解吸曲线。这种两步行为可能是由于Mg 2(dobpdc)的不对称六边形孔引起的氨基甲酸铵链之间的空间相互作用所致,导致潮湿条件下CO 2的工作能力降低和水对水的共同吸附增加。为了最大程度地减少这些不利的空间相互作用,我们针对了等规网状扩展骨架Mg 2的二胺附加变体(dotpdc)(dotpdc 4- = 4,4'' - dioxido- [1,1':4',1'' -三联苯] -3,3'' -二羧酸酯),这里报告首次和以前报道的异构构架Mg-IRMOF-74-II或Mg 2(pc-dobpdc)(pc-dobpdc 4- = 3,3'-dioxidobiphenyl-4,4'-dicarboxylate,pc =对-羧酸盐),其中与Mg 2(dobpdc)相反,它具有均匀的六角形孔。通过最小化氨基甲酸铵链之间的空间相互作用,这些框架可在所有情况下实现单个CO 2吸附/解吸步骤,以及减少水共吸附和提高对二胺损失的稳定性。Mg 2(pc-dobpdc)与大型二胺(如N)的官能化-(正庚基)乙二胺具有最佳的吸附性能,突出了调节孔的形状和二胺大小的优势,从而开发出了用于碳捕集应用的新型吸附剂。
更新日期:2017-11-03
中文翻译:

在Mg 2(dobpdc)的笨重的二胺附加变体中,克服了CO 2的两步吸附并最大程度地减少了水的共吸附
金属-有机骨架Mg 2(dobpdc)(dobpdc 4- = 4,4'-dioxidobiphenyl-3,3'-dicarboxylate)的烷基二胺官能化变体因其独特的阶梯状CO 2而有望用于CO 2捕集应用。氨基甲酸铵链协同形成的吸附曲线。伯,仲(1°,2°)烷基乙二胺附加变体因其CO 2低而特别受关注阶跃压力(在40°C时≤1mbar),最小的吸附/解吸滞后和高热稳定性。在本文中,我们证明了进一步增加仲胺上烷基的尺寸提供了增强的抵抗二胺挥发的稳定性,而且还导致了令人惊讶的两步式CO 2吸附/解吸曲线。这种两步行为可能是由于Mg 2(dobpdc)的不对称六边形孔引起的氨基甲酸铵链之间的空间相互作用所致,导致潮湿条件下CO 2的工作能力降低和水对水的共同吸附增加。为了最大程度地减少这些不利的空间相互作用,我们针对了等规网状扩展骨架Mg 2的二胺附加变体(dotpdc)(dotpdc 4- = 4,4'' - dioxido- [1,1':4',1'' -三联苯] -3,3'' -二羧酸酯),这里报告首次和以前报道的异构构架Mg-IRMOF-74-II或Mg 2(pc-dobpdc)(pc-dobpdc 4- = 3,3'-dioxidobiphenyl-4,4'-dicarboxylate,pc =对-羧酸盐),其中与Mg 2(dobpdc)相反,它具有均匀的六角形孔。通过最小化氨基甲酸铵链之间的空间相互作用,这些框架可在所有情况下实现单个CO 2吸附/解吸步骤,以及减少水共吸附和提高对二胺损失的稳定性。Mg 2(pc-dobpdc)与大型二胺(如N)的官能化-(正庚基)乙二胺具有最佳的吸附性能,突出了调节孔的形状和二胺大小的优势,从而开发出了用于碳捕集应用的新型吸附剂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号