当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Quantitative Assessment of Drug Delivery to Tissues and Association with Phospholipidosis: A Case Study with Two Structurally Related Diamines in Development
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00480 Irena Loryan 1 , Edmund Hoppe 2 , Klaus Hansen 2 , Felix Held 3, 4 , Achim Kless 2 , Klaus Linz 2 , Virginia Marossek 2 , Bert Nolte 2 , Paul Ratcliffe 2 , Derek Saunders 2 , Rolf Terlinden 2 , Anita Wegert 2 , André Welbers 2 , Olaf Will 2 , Margareta Hammarlund-Udenaes 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00480 Irena Loryan 1 , Edmund Hoppe 2 , Klaus Hansen 2 , Felix Held 3, 4 , Achim Kless 2 , Klaus Linz 2 , Virginia Marossek 2 , Bert Nolte 2 , Paul Ratcliffe 2 , Derek Saunders 2 , Rolf Terlinden 2 , Anita Wegert 2 , André Welbers 2 , Olaf Will 2 , Margareta Hammarlund-Udenaes 1
Affiliation
|
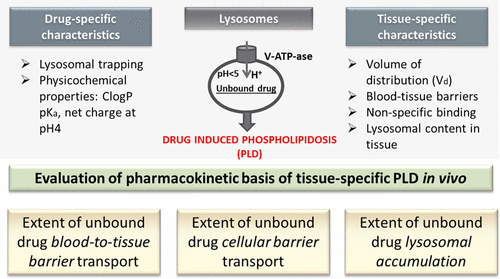
Drug induced phospholipidosis (PLD) may be observed in the preclinical phase of drug development and pose strategic questions. As lysosomes have a central role in pathogenesis of PLD, assessment of lysosomal concentrations is important for understanding the pharmacokinetic basis of PLD manifestation and forecast of potential clinical appearance. Herein we present a systematic approach to provide insight into tissue-specific PLD by evaluation of unbound intracellular and lysosomal (reflecting acidic organelles) concentrations of two structurally related diprotic amines, GRT1 and GRT2. Their intratissue distribution was assessed using brain and lung slice assays. GRT1 induced PLD both in vitro and in vivo. GRT1 showed a high intracellular accumulation that was more pronounced in the lung, but did not cause cerebral PLD due to its effective efflux at the blood–brain barrier. Compared to GRT1, GRT2 revealed higher interstitial fluid concentrations in lung and brain, but more than 30-fold lower lysosomal trapping capacity. No signs of PLD were seen with GRT2. The different profile of GRT2 relative to GRT1 is due to a structural change resulting in a reduced basicity of one amino group. Hence, by distinct chemical modifications, undesired lysosomal trapping can be separated from desired drug delivery into different organs. In summary, assessment of intracellular unbound concentrations was instrumental in delineating the intercompound and intertissue differences in PLD induction in vivo and could be applied for identification of potential lysosomotropic compounds in drug development.
中文翻译:

定量评估药物向组织的输送以及与磷脂病的关系:两种在发展中与结构相关的二胺的案例研究
在药物开发的临床前阶段可能会观察到药物诱发的磷脂酰肌病(PLD),并提出了战略性的问题。由于溶酶体在PLD的发病机理中起着核心作用,因此溶酶体浓度的评估对于理解PLD表现的药代动力学基础和预测潜在的临床表现非常重要。本文中,我们介绍了一种系统的方法,可通过评估两种结构相关的双质子胺GRT1和GRT2的未结合细胞内和溶酶体(反映酸性细胞器)浓度来了解组织特异性PLD。使用脑和肺切片测定法评估了它们的组织内分布。GRT1在体外和体内均可诱导PLD。GRT1显示出较高的细胞内蓄积,在肺中更为明显,但由于其在血脑屏障上的有效流出而不会引起脑部PLD。与GRT1相比,GRT2在肺和脑中显示出较高的组织液浓度,但溶酶体捕获能力却降低了30倍以上。GRT2未见PLD征象。GRT2相对于GRT1的差异是由于结构变化导致一个氨基的碱性降低。因此,通过不同的化学修饰,可以将不需要的溶酶体捕获与所需的药物递送到不同器官中分开。总之,评估细胞内未结合浓度有助于描述体内PLD诱导过程中化合物之间和组织间的差异 可以用于鉴定药物开发中潜在的溶同溶性化合物。
更新日期:2017-11-03
中文翻译:

定量评估药物向组织的输送以及与磷脂病的关系:两种在发展中与结构相关的二胺的案例研究
在药物开发的临床前阶段可能会观察到药物诱发的磷脂酰肌病(PLD),并提出了战略性的问题。由于溶酶体在PLD的发病机理中起着核心作用,因此溶酶体浓度的评估对于理解PLD表现的药代动力学基础和预测潜在的临床表现非常重要。本文中,我们介绍了一种系统的方法,可通过评估两种结构相关的双质子胺GRT1和GRT2的未结合细胞内和溶酶体(反映酸性细胞器)浓度来了解组织特异性PLD。使用脑和肺切片测定法评估了它们的组织内分布。GRT1在体外和体内均可诱导PLD。GRT1显示出较高的细胞内蓄积,在肺中更为明显,但由于其在血脑屏障上的有效流出而不会引起脑部PLD。与GRT1相比,GRT2在肺和脑中显示出较高的组织液浓度,但溶酶体捕获能力却降低了30倍以上。GRT2未见PLD征象。GRT2相对于GRT1的差异是由于结构变化导致一个氨基的碱性降低。因此,通过不同的化学修饰,可以将不需要的溶酶体捕获与所需的药物递送到不同器官中分开。总之,评估细胞内未结合浓度有助于描述体内PLD诱导过程中化合物之间和组织间的差异 可以用于鉴定药物开发中潜在的溶同溶性化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号