当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Temperature/pH/Enzyme Triple-Responsive Cationic Protein/PAA-b-PNIPAAm Nanogels for Controlled Anticancer Drug and Photosensitizer Delivery against Multidrug Resistant Breast Cancer Cells
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00737 Trong-Ming Don,Kun-Ying Lu,Li-Jie Lin,Chun-Hua Hsu,Jui-Yu Wu,Fwu-Long Mi
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2017-11-03 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b00737 Trong-Ming Don,Kun-Ying Lu,Li-Jie Lin,Chun-Hua Hsu,Jui-Yu Wu,Fwu-Long Mi
|
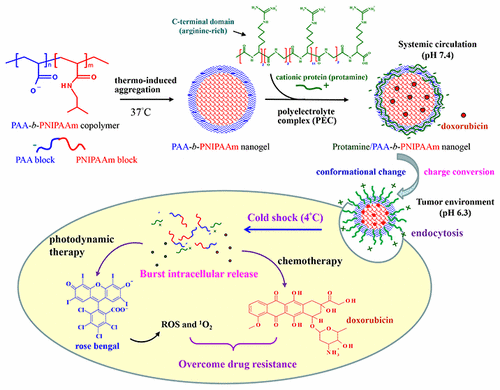
The tumor microenvironments are often acidic and overexpress specific enzymes. In this work, we synthesized a poly(AA-b-NIPAAm) copolymer (PAA-b-PNIPAAm) using a reversible addition–fragmentation chain transfer (RAFT) polymerization method. PAA-b-PNIPAAm and a cationic protein (protamine) were self-assembled into nanogels, which effectively reduced the cytotoxicity of protamine. The protamine/PAA-b-PNIPAAm nanogels were responsive to the stimuli including temperature, pH, and enzyme due to disaggregation of PAA-b-PNIPAAm, change in random coil/α-helix conformation of protamine, and enzymatic hydrolysis of the protein. Changing the pH from 7.4 to a lowered pHe (6.5–5.0) resulted in an increase in mean particle size and smartly converted surface charge from negative to positive. The cationic nanogels easily passed through the cell membrane and enhanced intracellular localization and accumulation of doxorubicin-loaded nanogels in multidrug resistant MCF-7/ADR breast cancer cells. Cold shock treatment triggered rapid intracellular release of doxorubicin against P-glycoprotein (Pgp)-mediated drug efflux, showing significantly improved anticancer efficacy as compared with free DOX. Furthermore, the nanogels were able to carry a rose bengal photosensitizer and caused significant damage to the multidrug resistant cancer cells under irradiation. The cationic nanogels with stimuli-responsive properties show promise as drug carrier for chemotherapy and photodynamic therapy against cancers.
中文翻译:

温度/ pH /酶三响应阳离子蛋白/ PAA- b -PNIPAAm纳米凝胶,可控制抗癌药物和对多药耐药性乳腺癌细胞的光敏剂递送
肿瘤微环境通常是酸性的,并且过表达特定的酶。在这项工作中,我们使用可逆的加成-断裂链转移(RAFT)聚合方法合成了聚(AA- b -NIPAAm)共聚物(PAA- b -PNIPAAm)。PAA- b- PNIPAAm和一种阳离子蛋白(鱼精蛋白)可自组装成纳米凝胶,可有效降低鱼精蛋白的细胞毒性。鱼精蛋白/ PAA- b -PNIPAAm纳米凝胶是响应于所述刺激包括温度,pH和酶由于PAA-的解聚b-PNIPAAm,鱼精蛋白无规卷曲/α-螺旋构象变化和蛋白质的酶促水解。将pH从7.4更改为更低的pHe(6.5–5.0)会导致平均粒径的增加,并且表面电荷会智能地从负值转换为正值。阳离子纳米凝胶易于穿过细胞膜,并在多药耐药的MCF-7 / ADR乳腺癌细胞中增强了阿霉素负载的纳米凝胶的细胞内定位和积累。冷休克治疗触发了阿霉素针对P糖蛋白(Pgp)介导的药物流出的快速细胞内释放,与游离DOX相比,显示出显着改善的抗癌功效。此外,纳米凝胶能够携带玫瑰孟加拉光敏剂,并在辐照下对耐多药癌细胞产生重大损害。
更新日期:2017-11-03
中文翻译:

温度/ pH /酶三响应阳离子蛋白/ PAA- b -PNIPAAm纳米凝胶,可控制抗癌药物和对多药耐药性乳腺癌细胞的光敏剂递送
肿瘤微环境通常是酸性的,并且过表达特定的酶。在这项工作中,我们使用可逆的加成-断裂链转移(RAFT)聚合方法合成了聚(AA- b -NIPAAm)共聚物(PAA- b -PNIPAAm)。PAA- b- PNIPAAm和一种阳离子蛋白(鱼精蛋白)可自组装成纳米凝胶,可有效降低鱼精蛋白的细胞毒性。鱼精蛋白/ PAA- b -PNIPAAm纳米凝胶是响应于所述刺激包括温度,pH和酶由于PAA-的解聚b-PNIPAAm,鱼精蛋白无规卷曲/α-螺旋构象变化和蛋白质的酶促水解。将pH从7.4更改为更低的pHe(6.5–5.0)会导致平均粒径的增加,并且表面电荷会智能地从负值转换为正值。阳离子纳米凝胶易于穿过细胞膜,并在多药耐药的MCF-7 / ADR乳腺癌细胞中增强了阿霉素负载的纳米凝胶的细胞内定位和积累。冷休克治疗触发了阿霉素针对P糖蛋白(Pgp)介导的药物流出的快速细胞内释放,与游离DOX相比,显示出显着改善的抗癌功效。此外,纳米凝胶能够携带玫瑰孟加拉光敏剂,并在辐照下对耐多药癌细胞产生重大损害。









































 京公网安备 11010802027423号
京公网安备 11010802027423号