当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two-Dimensional Arrangements of Bis(haloethynyl)benzenes Combining Halogen and Hydrogen Interactions
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.cgd.7b00690 Lucía González 1 , Rosa María Tejedor 2 , Eva Royo 1 , Blanca Gaspar 1 , Julen Munárriz 3, 4 , Anjana Chanthapally 5 , José Luis Serrano 6, 7 , Jagadese J. Vittal 5 , Santiago Uriel 1
Crystal Growth & Design ( IF 3.8 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.cgd.7b00690 Lucía González 1 , Rosa María Tejedor 2 , Eva Royo 1 , Blanca Gaspar 1 , Julen Munárriz 3, 4 , Anjana Chanthapally 5 , José Luis Serrano 6, 7 , Jagadese J. Vittal 5 , Santiago Uriel 1
Affiliation
|
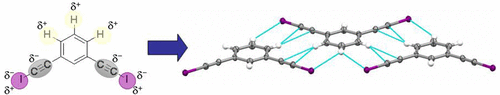
The electronic distribution of some haloethynylbenzene derivatives may favor the formation of two-dimensional organizations by combining halogen and hydrogen bonds. In order to highlight this strategy, we have prepared seven cocrystals and analyzed their structures. 1,4-Bis(iodoethynyl)benzene, 1,4-bis(bromoethynyl)benzene, and 1,3-bis(iodoethynyl)benzene were used as halogen bond donors and 1,2-bis(4-pyridyl)ethylene, pyridazine, propanone, hexamethylenetetramine, and 2,8-dimethyl-6H,12H-5,11-methanodibenzo[b,f][1,5]diazocine (Tröger’s base) were employed as halogen bond acceptors. The crystal structures of seven halogen-bonded complexes show C–X···Y (X = I, Br; Y = N, O) distances shorter than the sum of the van der Waals radii, and six of them contain the edge-to-edge C–H···X (X = I, Br) supramolecular hydrogen bond synthon. The stabilization energies with basis set superposition error correction of hydrogen bond synthons have been determined by DFT calculations, and they are in the range 2.9 to 5.7 kcalmol–1. To gain a deeper understanding of these interactions, noncovalent interaction methodology was also applied.
中文翻译:

卤和氢相互作用结合的双(卤乙炔基)苯的二维排列
一些卤代乙炔基苯衍生物的电子分布可通过结合卤素和氢键来促进二维组织的形成。为了突出此策略,我们准备了七个共晶并分析了它们的结构。1,4-双(碘乙炔基)苯,1,4-双(溴乙炔基)苯和1,3-双(碘乙炔基)苯用作卤素键供体,1,2-双(4-吡啶基)乙烯,哒嗪,丙酮,六亚甲基四胺和2,8-二甲基-6 H,12 H -5,11-甲基二苯并[ b,f] [1,5]重氮电影(特罗格氏碱)被用作卤素键受体。七个卤素键合配合物的晶体结构显示C–X··Y(X = I,Br; Y = N,O)距离短于范德华半径之和,并且其中六个包含边缘-边缘C–H···X(X = I,Br)超分子氢键合成子。已经通过DFT计算确定了具有氢键合子基数叠加误差校正的稳定能,范围为2.9至5.7 kcalmol –1。为了更深入地了解这些相互作用,还应用了非共价相互作用方法。
更新日期:2017-11-03
中文翻译:

卤和氢相互作用结合的双(卤乙炔基)苯的二维排列
一些卤代乙炔基苯衍生物的电子分布可通过结合卤素和氢键来促进二维组织的形成。为了突出此策略,我们准备了七个共晶并分析了它们的结构。1,4-双(碘乙炔基)苯,1,4-双(溴乙炔基)苯和1,3-双(碘乙炔基)苯用作卤素键供体,1,2-双(4-吡啶基)乙烯,哒嗪,丙酮,六亚甲基四胺和2,8-二甲基-6 H,12 H -5,11-甲基二苯并[ b,f] [1,5]重氮电影(特罗格氏碱)被用作卤素键受体。七个卤素键合配合物的晶体结构显示C–X··Y(X = I,Br; Y = N,O)距离短于范德华半径之和,并且其中六个包含边缘-边缘C–H···X(X = I,Br)超分子氢键合成子。已经通过DFT计算确定了具有氢键合子基数叠加误差校正的稳定能,范围为2.9至5.7 kcalmol –1。为了更深入地了解这些相互作用,还应用了非共价相互作用方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号