当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Alkyl Ether as a One-Carbon Synthon: Route to 2,4-Disubstituted 1,3,5-Triazines via C–H Amination/C–O Cleavage under Transition-Metal-Free Conditions
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.orglett.7b03171 Yizhe Yan 1, 2 , Zheng Li 1 , Hongyi Li 1 , Chang Cui 1 , Miaomiao Shi 1 , Yanqi Liu 1
Organic Letters ( IF 4.9 ) Pub Date : 2017-11-02 00:00:00 , DOI: 10.1021/acs.orglett.7b03171 Yizhe Yan 1, 2 , Zheng Li 1 , Hongyi Li 1 , Chang Cui 1 , Miaomiao Shi 1 , Yanqi Liu 1
Affiliation
|
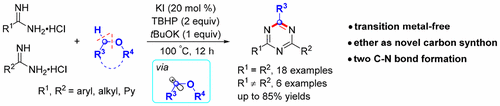
A transition-metal-free oxidative formal [3 + 2 + 1] cycloaddition for the synthesis of symmetrical and unsymmetrical 2,4-disubstituted 1,3,5-triazines has been first demonstrated. The reaction is involved in a domino C–H amination of alkyl ethers with amidines, C–O cleavage, nucleophilic addition, condensation, and an oxidative aromatization process. Notably, two C–N bonds were constructed in one pot, and alkyl ethers were employed as a novel carbon source of 1,3,5-triazines. The preliminary mechanistic studies revealed the reaction underwent a radical pathway.
中文翻译:

烷基醚作为单碳合成子:在无过渡金属的条件下通过C–H胺化/ C–O裂解反应生成2,4-二取代的1,3,5-三嗪
首次证明了用于合成对称和不对称的2,4-二取代的1,3,5-三嗪的无过渡金属氧化式[3 + 2 + 1]环加成反应。该反应涉及烷基醚与am的多米诺骨化CH胺化,CO裂解,亲核加成,缩合和氧化芳构化过程。值得注意的是,在一个罐中构建了两个C–N键,并且烷基醚被用作1,3,5-三嗪的新型碳源。初步的机理研究表明该反应经历了一个自由基途径。
更新日期:2017-11-02
中文翻译:

烷基醚作为单碳合成子:在无过渡金属的条件下通过C–H胺化/ C–O裂解反应生成2,4-二取代的1,3,5-三嗪
首次证明了用于合成对称和不对称的2,4-二取代的1,3,5-三嗪的无过渡金属氧化式[3 + 2 + 1]环加成反应。该反应涉及烷基醚与am的多米诺骨化CH胺化,CO裂解,亲核加成,缩合和氧化芳构化过程。值得注意的是,在一个罐中构建了两个C–N键,并且烷基醚被用作1,3,5-三嗪的新型碳源。初步的机理研究表明该反应经历了一个自由基途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号