European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.ejmech.2017.10.063 Laura L. Romero-Hernández , Penélope Merino-Montiel , Socorro Meza-Reyes , José Luis Vega-Baez , Óscar López , José M. Padrón , Sara Montiel-Smith
|
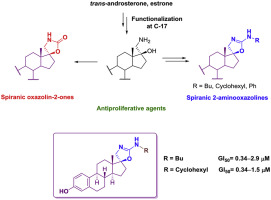
Herein we report the straightforward preparation of novel conformationally-restricted steroids from trans-androsterone and estrone, decorated with spiranic oxazolidin-2-one or 2-aminooxazoline motifs at C-17 as potential antiproliferative agents. Such unprecedented pharmacophores were accessed using an aminomethylalcohol derivative at C-17 as the key intermediate; reaction of such functionality with triphosgene, or conversion into N-substituted thioureas, followed by an intramolecular cyclodesulfurization reaction promoted by yellow HgO, furnished such spirocycles in excellent yields.
Title compounds were tested in vitro against a panel of six human tumor cell lines, named A549 (non-small cell lung), HBL-100 (breast), HeLa (cervix), SW1573 (non-small cell lung), T-47D (breast) and WiDr (colon), and the results were compared with steroidal chemotherapeutic agents (abiraterone and galeterone); the A-ring of the steroidal backbone, the nature of the heterocycle and the N-substituents proved to be essential motifs for establishing structure-activity relationships concerning not only the potency but also the selectivity against tumor cell lines. Estrone derivatives, particularly those bearing a spiranic 2-aminooxazoline scaffold were found to be the most active compounds, with GI50 values ranging from the low micromolar to the submicromolar level (0.34–1.5 μM). Noteworthy, the lead compounds showed a remarkable increase in activity against the resistant cancer cell lines (T-47D and WiDr) compared to the anticancer reference drugs (up to 120-fold).
中文翻译:

合成史无前例的甾体螺杂环化合物作为潜在的抗增殖药
本文中我们报道了由反式雄甾酮和雌酮直接构象限制的类固醇的直接制备,在C-17处用吡喃恶唑烷-2-或2-氨基恶唑啉基作为装饰物作为潜在的抗增殖剂。使用C-17处的氨基甲基醇衍生物作为关键中间体,可以得到这种空前的药效团。该官能团与三光气反应,或转化成N-取代的硫脲,然后由黄色HgO促进的分子内环脱硫反应,以优异的产率提供了这种螺环。
标题化合物针对一组六种人类肿瘤细胞系进行了体外测试,分别命名为A549(非小细胞肺),HBL-100(乳腺),HeLa(子宫颈),SW1573(非小细胞肺),T-47D (乳房)和WiDr(冒号),并将结果与类固醇化疗药物(阿比特龙和加利泰隆)进行比较;甾体主链的A环,杂环的性质和N-取代基被证明是建立结构-活性关系的基本主题,这些关系不仅涉及效力,还涉及对肿瘤细胞系的选择性。发现雌酮衍生物,尤其是带有螺氨基2-氨基恶唑啉骨架的那些是活性最高的化合物,其GI 50值范围从低微摩尔到亚微摩尔水平(0.34–1.5μM)。值得注意的是,与抗癌参考药物相比,先导化合物对耐药性癌细胞系(T-47D和WiDr)的活性显着提高(高达120倍)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号