当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Z-Selective iridium-catalyzed cross-coupling of allylic carbonates and α-diazo esters
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7sc04283c Bryce N. Thomas 1, 2, 3, 4 , Patrick J. Moon 1, 2, 3, 4 , Shengkang Yin 1, 2, 3, 4 , Alex Brown 1, 2, 3, 4 , Rylan J. Lundgren 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2017-10-24 00:00:00 , DOI: 10.1039/c7sc04283c Bryce N. Thomas 1, 2, 3, 4 , Patrick J. Moon 1, 2, 3, 4 , Shengkang Yin 1, 2, 3, 4 , Alex Brown 1, 2, 3, 4 , Rylan J. Lundgren 1, 2, 3, 4
Affiliation
|
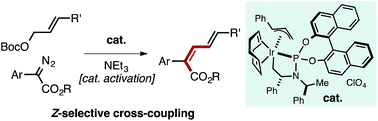
A well-defined Ir–allyl complex catalyzes the Z-selective cross-coupling of allyl carbonates with α-aryl diazo esters. The process overrides the large thermodynamic preference for E-products typically observed in metal-mediated coupling reactions to enable the synthesis of Z,E-dieneoates in good yield with selectivities consistently approaching or greater than 90 : 10. This transformation represents the first productive merger of Ir–carbene and Ir–allyl species, which are commonly encountered intermediates in allylation and cyclopropanation/E–H insertion catalysis. Potentially reactive functional groups (aryl halides, ketones, nitriles, olefins, amines) are tolerated owing to the mildness of reaction conditions. Kinetic analysis of the reaction suggests oxidative addition of the allyl carbonate to an Ir-species is rate-determining. Mechanistic studies uncovered a pathway for catalyst activation mediated by NEt3.
中文翻译:

Z-铱催化的烯丙基碳酸酯和α-重氮酯的交叉偶联
定义明确的Ir-烯丙基络合物催化碳酸烯丙酯与α-芳基重氮酯的Z选择性交叉偶联。该方法优先于金属介导的偶联反应中通常观察到的E产物的热力学偏好,以实现Z,E的合成。-二烯高产率,选择性始终接近或大于90:10。这种转化代表了Ir-卡宾和Ir-烯丙基物种的首次生产合并,这是烯丙基化和环丙烷化/ E-H插入催化中经常遇到的中间体。由于反应条件温和,因此可以耐受潜在的反应性官能团(卤代芳基,酮,腈,烯烃,胺)。反应的动力学分析表明碳酸烯丙酯向Ir物种的氧化加成是决定速率的。机理研究揭示了NEt 3介导的催化剂活化途径。
更新日期:2017-11-02
中文翻译:

Z-铱催化的烯丙基碳酸酯和α-重氮酯的交叉偶联
定义明确的Ir-烯丙基络合物催化碳酸烯丙酯与α-芳基重氮酯的Z选择性交叉偶联。该方法优先于金属介导的偶联反应中通常观察到的E产物的热力学偏好,以实现Z,E的合成。-二烯高产率,选择性始终接近或大于90:10。这种转化代表了Ir-卡宾和Ir-烯丙基物种的首次生产合并,这是烯丙基化和环丙烷化/ E-H插入催化中经常遇到的中间体。由于反应条件温和,因此可以耐受潜在的反应性官能团(卤代芳基,酮,腈,烯烃,胺)。反应的动力学分析表明碳酸烯丙酯向Ir物种的氧化加成是决定速率的。机理研究揭示了NEt 3介导的催化剂活化途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号